Notice: Because of their
potential importance in the treatment of Lyme disease, these articles are being
published early (on June 12, 2001). The final versions will appear in the July
12 issue of the Journal.
![]()
Prophylaxis with
Single-Dose Doxycycline for the Prevention of Lyme Disease after an Ixodes scapularis Tick Bite
Robert B. Nadelman, M.D., John Nowakowski, M.D., Durland
Fish, Ph.D., Richard C. Falco, Ph.D., Katherine Freeman, Dr.P.H., Donna
McKenna, R.N., Peter Welch, M.D., Robert Marcus, M.D., Maria E.
Agüero-Rosenfeld, M.D., David T. Dennis, M.D., and Gary P. Wormser, M.D., for
the Tick Bite Study Group [ Note
]
ABSTRACT
Background It is
unclear whether antimicrobial treatment after an Ixodes scapularis tick bite will prevent Lyme disease.
Methods In an area
of New York where Lyme disease is hyperendemic we conducted a randomized,
double-blind, placebo-controlled trial of treatment with a single 200-mg dose
of doxycycline in 482 subjects who had removed attached I. scapularis ticks from their bodies
within the previous 72 hours. At base line, three weeks, and six weeks,
subjects were interviewed and examined, and serum antibody tests were
performed, along with blood cultures for Borrelia
burgdorferi. Entomologists confirmed the species of the ticks and
classified them according to sex, stage, and degree of engorgement.
Results Erythema
migrans developed at the site of the tick bite in a significantly smaller
proportion of the subjects in the doxycycline group than of those in the
placebo group (1 of 235 subjects [0.4 percent] vs. 8 of 247 subjects [3.2
percent], P<0.04). The efficacy of treatment was 87 percent (95 percent
confidence interval, 25 to 98 percent). Objective extracutaneous signs of Lyme
disease did not develop in any subject, and there were no asymptomatic
seroconversions. Treatment with doxycycline was associated with more frequent
adverse effects (in 30.1 percent of subjects, as compared with 11.1 percent of
those assigned to placebo; P<0.001), primarily nausea (15.4 percent vs. 2.6
percent) and vomiting (5.8 percent vs. 1.3 percent). Erythema migrans developed
more frequently after untreated bites from nymphal ticks than after bites from
adult female ticks (8 of 142 bites [5.6 percent] vs. 0 of 97 bites [0 percent],
P=0.02) and particularly after bites from nymphal ticks that were at least
partially engorged with blood (8 of 81 bites [9.9 percent], as compared with 0
of 59 bites from unfed, or flat, nymphal ticks [0 percent]; P=0.02).
Conclusions A single
200-mg dose of doxycycline given within 72 hours after an I. scapularis tick bite can prevent the
development of Lyme disease.
Notice: Because of their
potential importance in the treatment of Lyme disease, these articles are being
published early (on June 12, 2001). The final versions will appear in the July
12 issue of the Journal.
Lyme disease is transmitted by the bite of an Ixodes scapularis tick and is the most
common vector-borne disease in the United States.1
This infection may be prevented by vaccination.2
3
However, the vaccine's general acceptance is likely to be limited by its cost
(a cost to the pharmacist of $61.25 per dose) and the need for multiple doses
to achieve and maintain protection.2
3
In addition, the vaccine is less than 100 percent effective and is currently
approved only for persons 15 to 70 years of age.3
Antimicrobial prophylaxis for persons with I. scapularis tick bites may be a way to
prevent Lyme disease. However, it is not known whether antimicrobial agents can
effectively cure incubating Borrelia
burgdorferi infection. In an animal model of another tick-borne
disease, Rocky Mountain spotted fever, antibiotic prophylaxis appeared to delay
but not prevent infection.4
Antimicrobial therapy for the prevention of Lyme disease after I. scapularis tick bites has not been
shown to be effective in controlled treatment trials.5
6
7
8
9
In these studies, as well as in a model of cost effectiveness,10
the drug regimens consisted of courses of antibiotics lasting 10 to 14 days,
similar to those typically recommended for the treatment of clinically evident
early Lyme disease. On the basis of the experience with syphilis11
and leptospirosis,12
it might be anticipated, however, that a much shorter course of antimicrobial
therapy would be effective in treating an incubating (but inapparent)
spirochetal infection. We studied the efficacy and safety of a single 200-mg
dose of doxycycline in preventing Lyme disease after an I. scapularis tick bite.
Methods
Subjects
Between May 1987 and December 1996, we recruited subjects who had
removed an attached I. scapularis
tick from their bodies within the preceding 72 hours and had been bitten in
Westchester County, New York, where Lyme disease is hyperendemic.13
Eligible subjects 12 years old or older were enrolled after they had given
written informed consent. Parental consent was obtained for those who were
younger than 18 years old. Subjects were excluded if they had clinical signs of
Lyme disease (e.g., erythema migrans) at the time of enrollment, were taking or
had just completed a course of antibiotics effective against B. burgdorferi, were pregnant or lactating,
had been vaccinated against Lyme disease, or did not submit to study personnel
the tick that bit them. Enrolled subjects whose ticks were later identified as
something other than I. scapularis
were included only in the analysis of safety. Subjects were evaluated at
Westchester Medical Center, a university medical center (461 of the 506
subjects [91.1 percent]), or at a nearby community hospital (45 subjects [8.9
percent]).
Ticks
The species, sex, and stage of the ticks were determined by a
medical entomologist. Ticks were initially classified as unfed (flat) or partly
fed (partially engorged) on the basis of a visual inspection. When possible,
the duration of the tick's attachment to the subject was estimated on the basis
of a measurement of the tick scutal index. This determination (the ratio of
tick body length to scutal width) was calculated as reported previously.14
Clinical Evaluation
At enrollment, at three weeks, and at six weeks, participants were
examined and interviewed with the use of a written questionnaire. During the
course of the study, specific questions regarding adverse effects of the study
medication were added to the questionnaire. The analysis of adverse events was
therefore restricted to the 309 subjects for whom this information was
available. Subjects were encouraged to contact study personnel if clinical
symptoms occurred between the scheduled visits or in the period immediately
after the final visit. They were also counseled on ways to prevent tick bites.
Blood was collected at each visit for antibody testing and for culture for B. burgdorferi.
Study Medication
After clinical evaluation and phlebotomy, subjects were given two
pills from a vial that contained either two 100-mg capsules of doxycycline or
two identical-appearing placebo pills containing lactose. Capsules were prepared
by the hospital pharmacy and distributed according to a randomization list that
maintained a 1:1 ratio between subjects in the doxycycline group and those in
the placebo group. Both subjects and study personnel were blinded to the
contents of the vials. Subjects swallowed the pills under direct observation by
study personnel.
Laboratory Tests
Urine pregnancy tests (Clearview HCG II, Wampole Laboratories,
Cranbury, N.J.) were performed at the initial encounter for all women of
childbearing potential. Serum antibodies to B.
burgdorferi were measured by polyvalent fluorescent immunoassay
(FIAX, Whittaker Bioproducts, Walkersville, Md.) from 1987 through 1990, and by
polyvalent enzyme-linked immunosorbent assay (ELISA) (WhittakerStat, Whittaker
Bioproducts) after 1990. Specimens with equivocal or positive assay results
were retested by separate immunoblot assays for IgM and IgG antibodies to B. burgdorferi (MarDx Diagnostics,
Carlsbad, Calif.). All tests were performed and interpreted according to the manufacturers'
instructions. Assays on specimens from the same patient were run in parallel.
Heparinized whole blood (0.3 ml) or, in some cases, serum (0.3 ml) was cultured
for B. burgdorferi in modified
Barbour–Stoenner–Kelly medium by means of previously described techniques.15
Primary End Point
The primary end point was the development of erythema migrans at
the site of the tick bite. Erythema migrans occurring at a different site from
that of the identified tick bite and laboratory evidence of B. burgdorferi infection in the absence of
erythema migrans were analyzed as secondary end points. Seroconversion was
defined as a change from a negative result on ELISA to an equivocal or positive
result in association with the presence of IgM bands on immunoblotting that met
the recommended criteria for seropositivity.16
Sample Size
The frequency of Lyme disease (characterized by erythema migrans)
among untreated subjects who had been bitten by an I. scapularis tick in Westchester County was initially
estimated to be approximately 5 percent. The smallest clinically important
reduction in this rate was considered to be a reduction from 5 percent to 1
percent. Since it was expected that doxycycline would be at least as effective
as placebo in preventing the occurrence of disease, the hypothesis was
considered one-tailed. Because the frequency (incidence) in each group was
expected to be quite small, an arc-sine transformation was performed in
conjunction with the binomial test for two independent samples to derive the
required sample sizes. On the basis of an alpha level of 0.05 and a power of 80
percent, the planned sample size was 129 subjects in each treatment group. At
the time the projected number of subjects had been enrolled, it appeared that
the risk of erythema migrans was limited to subjects who had been bitten by
nymphal I. scapularis ticks. Thus,
it became important to continue to enroll subjects until sufficient statistical
power could be achieved in the subgroup of subjects bitten by nymphal ticks.
Statistical Analysis
Categorical variables were compared by means of the two-tailed
Fisher's exact test or the two-tailed chi-square test. The final analysis for
the primary end point was also two-tailed, in order to be more conservative.
Student's t-test was used for continuous variables. Statistical analyses were
performed with the use of SAS software (version 6.12, SAS Institute, Cary,
N.C.). Because an interim analysis was performed in September 1992, the
determination of the alpha level was based on the O'Brien–Fleming criteria.17
A P value of 0.0475 or lower was considered to indicate statistical
significance in the final analysis. The efficacy of prophylaxis was calculated
as follows: (1–[the risk of infection among the doxycycline-treated
subjects÷the risk among subjects receiving placebo])×100 percent.8
A 95 percent confidence interval was computed around the efficacy rate with the
use of the test-based method.18
Results
A total of 506 subjects were randomly assigned to receive either
doxycycline or placebo; this total included 6 persons who were enrolled twice
in different years. The primary (intention-to-treat) analysis was restricted to
the 482 subjects who had removed identifiable I.
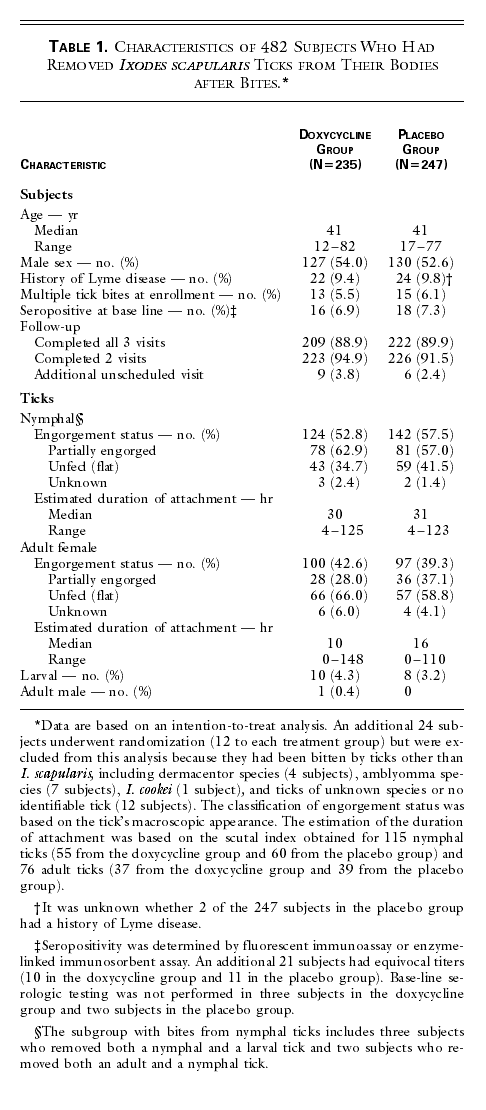
scapularis ticks from their bodies (Table
1). Of those subjects, 28 had removed multiple ticks at the time of the
bite that led to enrollment, including 23 who had removed at least two ticks of
the same stage, 3 who had removed both a nymphal and a larval I. scapularis tick, and 2 who had removed
both a nymphal and an adult I. scapularis
tick. (For certain analyses, the latter five subjects were included in the
subgroup of subjects who had removed only nymphal ticks.) The demographic
characteristics of the 235 subjects in the doxycycline group were similar to
those of the 247 subjects in the placebo group (Table
1). A total of 431 subjects (89.4 percent) completed all three visits
(enrollment, three weeks, and six weeks).
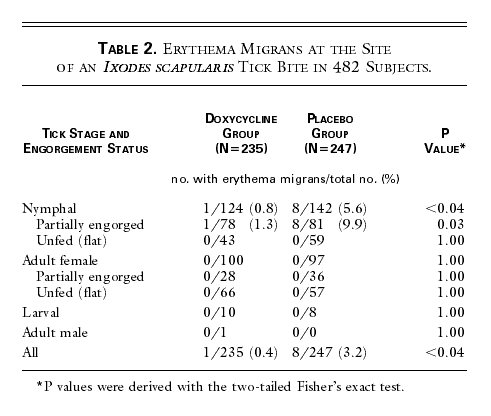
Erythema migrans occurred at the site of the tick bite in 8 of the
247 subjects in the placebo group (3.2 percent), as compared with 1 of the 235
subjects in the doxycycline group (0.4 percent, P<0.04). Seven of these nine
subjects also had laboratory evidence of Lyme disease, including skin cultures
positive for B. burgdorferi in
all four subjects who underwent a skin biopsy. Seroconversion determined by
ELISA occurred in seven subjects. An additional subject (in the doxycycline
group) who remained seronegative by ELISA was positive for IgM antibody on
immunoblotting. The last of the nine subjects with erythema migrans had an equivocal
result on ELISA and negative results for IgM and IgG antibodies on
immunoblotting and did not return for serologic testing during the
convalescence phase.
Erythema migrans developed at the site of the tick bite a median
of 12 days (range, 4 to 17) after the removal of nymphal I. scapularis ticks that showed visual
evidence of partial engorgement with blood (Table
2). In untreated subjects, bites from nymphal ticks were significantly more
likely than bites from adult ticks to be associated with erythema migrans (8 of
142 [5.6 percent] vs. 0 of 97 [0 percent], P=0.02).
In the two groups combined, nymphal ticks were nearly twice as
likely as adult ticks to be partially engorged (159 of 266 ticks [59.8 percent]
vs. 64 of 197 ticks [32.5 percent], P<0.001). The estimated median duration
of attachment, based on the tick scutal index for the 115 nymphal ticks that
were measured, was 30 hours (range, 4 to 125), as compared with 10 hours
(range, 0 to 148) for 76 adult ticks (P<0.001). Untreated bites from nymphal
ticks that had been attached to subjects for an estimated 72 hours or longer
were more likely to result in erythema migrans than were untreated bites from
nymphal ticks that had been feeding for less than 72 hours (3 of 12 bites [25
percent; 95 percent confidence interval, 7 to 57 percent] vs. 0 of 48,
P=0.006).
Objective extracutaneous manifestations of Lyme disease (e.g.,
facial-nerve palsy, meningitis, heart block, and oligoarthritis) were not
observed during the study period, nor was asymptomatic seroconversion (the
development of antibody to B. burgdorferi). However,
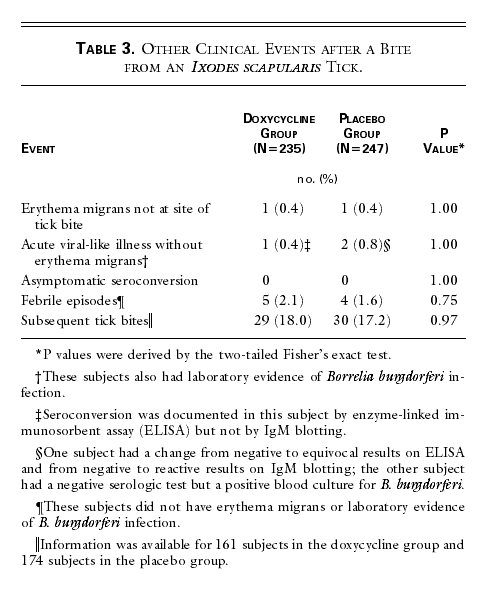
in addition to the nine subjects in whom erythema migrans developed at the
identified site of the tick bite, solitary erythema migrans lesions developed
in two subjects (one in each group) at other sites. In three other subjects
(one in the doxycycline group and two in the placebo group), transient
viral-like illnesses developed, with laboratory evidence of B. burgdorferi infection (Table
3).
Nine additional subjects (five in the doxycycline group and four
in the placebo group) reported febrile episodes after removing I. scapularis ticks during the six-week
study period but had no laboratory evidence of B.
burgdorferi infection. A total of 59 of the 325 subjects questioned
(18.2 percent) recognized additional tick bites after enrollment but during the
six-week study period.
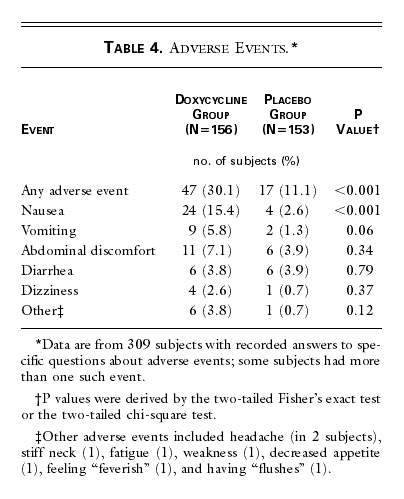
Adverse events (primarily nausea and vomiting) were more frequent
in the doxycycline group than in the placebo group (P<0.001) (Table
4). However, these events were not serious and were self-limited. No
subject reported photosensitivity or a rash attributable to the study
medication.
Discussion
This randomized, controlled trial shows that antimicrobial
prophylaxis with a single 200-mg dose of doxycycline, given after a recognized
bite from an I. scapularis tick,
is highly effective in preventing the development of Lyme disease. Prophylaxis
with doxycycline had an efficacy of 87 percent, which compares favorably with
the 95 percent efficacy rate of doxycycline given once weekly to prevent
leptospirosis.12
The efficacy rate found in our study should be interpreted cautiously, however,
because of the relatively small number of subjects in whom Lyme disease
developed and the resultant wide 95 percent confidence interval (25 to 98
percent).
Our results contrast with those of previous studies,6
7
8
which showed no clear protection attributable to antimicrobial prophylaxis
given after a tick bite. We observed a beneficial effect of prophylactic
doxycycline despite a fairly low infection rate in the placebo group (3.2
percent) — a rate similar to that in other studies (range, 1.1 to 3.4 percent).
The fact that our study demonstrated the efficacy of antimicrobial prophylaxis
is probably related to its size (482 subjects, as compared with 56 subjects,6
184 subjects,7
and 387 subjects8
in the other randomized studies), which provided the study with greater
statistical power to show relatively small differences.
Our use of a restrictive primary end point (erythema migrans at
the site of the tick bite) could have resulted in underestimation of the actual
incidence of B. burgdorferi
infection attributable to the bite of an identified I. scapularis tick. However, this end point was chosen
deliberately. Erythema migrans at the site of the bite is the most common
clinical manifestation associated with B.
burgdorferi infection and is the only reliable marker of infection
caused by that specific bite. As shown in our study, subsequent tick bites are
common (reported by 59 of the 325 subjects we questioned [18.2 percent]), even
over a period as short as six weeks. Indeed, solitary erythema migrans
developed in two of the study subjects at a site other than that of the initial
tick bite, suggesting the occurrence of an additional, unrecognized bite. The
follow-up was limited to six weeks in order to reduce confounding associated
with illnesses that might result from subsequent tick bites.
A theoretical risk associated with prophylactic antimicrobial
treatment is that it might alter the disease presentation so that the
characteristic erythema migrans rash might not be manifested in treated
subjects, in whom a more subtle, nonspecific illness might develop or
asymptomatic seroconversion might occur. In such circumstances, an unrecognized
latent infection might eventually result in arthritis or neurologic disease. We
believe that this is unlikely for several reasons. First, nonspecific febrile illnesses
were not disproportionately common in the doxycycline group. Furthermore, there
was no asymptomatic seroconversion (suggesting the occurrence of subclinical
infection) in subjects in the doxycycline group (or in the placebo group). In
addition, there was no delayed onset of erythema migrans at the original site
of the tick bite in any subject during the six weeks of observation — a period
four times the average incubation period for this rash.19
Finally, objective extracutaneous manifestations of Lyme disease did not
develop in any of the subjects in our study or in the three other prospective
trials of antimicrobial prophylaxis (with follow-up lasting between six months
and three years).6
7
8
Our finding that only ticks that are partially engorged with blood
are associated with the development of erythema migrans at the site of the bite
is consistent with studies in animals, which have demonstrated that B. burgdorferi is infrequently transmitted
before the tick has been attached for 48 hours.20
Our results also confirm those of Sood et al.,21
who found a significantly increased risk of B.
burgdorferi infection in humans after tick bites involving an
estimated duration of attachment of 72 hours or longer. In addition, our
findings support those of previous epidemiologic studies that have shown a
temporal association between the development of erythema migrans and exposure
to nymphal rather than adult ticks.13
One possible explanation for this is that adult ticks (which are considerably
larger than nymphal ticks) are detected and removed earlier in the feeding process
than nymphal ticks; in our study, the estimated median duration of attachment
for adult ticks in both groups (10 hours) was one third as long as that for
nymphal ticks (30 hours).
Although no serious adverse events were noted, 30.1 percent of
those who received doxycycline had medication-related problems, as compared
with 11.1 percent with placebo. The events reported were primarily nausea (15.4
percent with doxycycline vs. 2.6 percent with placebo, P<0.001) and vomiting
(5.8 percent vs. 1.3 percent, P=0.06). Taking doxycycline with food may improve
its tolerability, with only a minimal decrease in peak serum levels.12
The ticks in our study were identified by medical entomologists.
Patients and clinicians may have difficulty in distinguishing I. scapularis from other ticks and
arthropods, and even from scabs or debris.22
Furthermore, the efficacy of doxycycline in the prevention of other infections
transmitted by I. scapularis
ticks (e.g., babesiosis and human granulocytic ehrlichiosis) is unknown and
should not be assumed. Nor can it be assumed that other antimicrobial agents
that are effective for the treatment of Lyme disease (e.g., amoxicillin) or
even other regimens of doxycycline (e.g., 100 mg twice daily) would have
similar efficacy when used for short-term prophylaxis.
Table 1. Characteristics of 482 Subjects
Who Had Removed Ixodes scapularis
Ticks from Their Bodies after Bites.
|
Table 2. Erythema Migrans at the Site of
an Ixodes scapularis Tick Bite
in 482 Subjects.
|
Table 3. Other Clinical Events after a
Bite from an Ixodes scapularis
Tick.
|
Table 4. Adverse Events.
|
Source Information
From the Department of Medicine, Division of Infectious Diseases
(R.B.N., J.N., R.C.F., D.M., G.P.W.), and the Department of Pathology
(M.E.A.-R.), New York Medical College; and the Lyme Disease Diagnostic Center,
Westchester Medical Center (R.B.N., J.N., D.M., G.P.W.) — both in Valhalla,
N.Y.; the Department of Epidemiology and Public Health, Yale University School
of Medicine, New Haven, Conn. (D.F.); the Vector Ecology Laboratory, Louis
Calder Center, Fordham University, Armonk, N.Y. (R.C.F.); the Department of
Epidemiology and Social Medicine, Albert Einstein College of Medicine, Bronx,
N.Y. (K.F.); Northern Westchester Hospital Center, Mt. Kisco, N.Y. (P.W.,
R.M.); and the Division of Vector-Borne Infectious Diseases, National Center
for Infectious Diseases, Centers for Disease Control and Prevention, Fort
Collins, Colo. (D.T.D.). Address reprint requests to Dr. Nadelman at the
Division of Infectious Diseases, Westchester Medical Center, Macy Pavilion 209
Southeast, Valhalla, NY 10595.
Supported in part by grants from the Tick-Borne Diseases Institute
of the New York State Department of Health (C-003836, C-008372, C-011001, and
C-015088) and the Centers for Disease Control and Prevention (U50/CCU 210280
and U50/CCU 210286). The contents of this report are solely the responsibility
of the authors and do not necessarily represent the official views of the New
York State Department of Health or the Centers for Disease Control and
Prevention.
We are indebted to Kathleen O'Keefe, R.N., Harold Horowitz, M.D., Marisa
Montecalvo, M.D., Dominick Corbi, Dionysios Liveris, Ph.D., Thomas Daniels,
Ph.D., Rhonda Corda, Jane Rainaldi, R.N., Richard Ginther, David Labowitz,
Carol Carbonaro, Ph.D., Theresa Boccia, Erin McHugh, Paul Visintainer, Ph.D.,
and Daniel Byrne, M.S., for their assistance.
Footnotes
Other investigators in the Tick Bite Study Group are listed in the
Appendix.
References
1. Orloski KA, Hayes EB, Campbell GL, Dennis DT. Surveillance for
Lyme disease — United States, 1992–1998. MMWR CDC Surveill Summ
2000;49(SS-3):1-11.
[ Return
to Text ]
2. Steere AC, Sikand VK, Meurice F, et al. Vaccination against Lyme disease
with recombinant Borrelia burgdorferi outer-surface lipoprotein A with
adjuvant. N Engl J Med 1998;339:209-15.
[ Return
to Text ] [ Abstract
]
3. Lyme disease vaccine. Med Lett Drugs Ther 1999;41:29-30.
[ Return
to Text ]
4. Kenyon RH, Williams RG, Oster CN, Pedersen CE Jr. Prophylactic treatment of
Rocky Mountain spotted fever. J Clin Microbiol 1978;8:102-4.
[ Return
to Text ]
5. Wormser GP, Nadelman RB, Dattwyler RJ, et al. Practice guidelines for the
treatment of Lyme disease. Clin Infect Dis 2000;31:Suppl 1:1-14.
[ Return
to Text ]
6. Costello CM, Steere AC, Pinkerton RE, Feder HM Jr. A prospective study of
tick bites in an endemic area for Lyme disease. J Infect Dis 1989;159:136-9.
[ Return
to Text ]
7. Agre F, Schwartz R. The value of early treatment of deer tick bites for the
prevention of Lyme disease. Am J Dis Child 1993;147:945-7.
[ Return
to Text ]
8. Shapiro ED, Gerber MA, Holabird ND, et al. A controlled trial of
antimicrobial prophylaxis for Lyme disease after deer-tick bites. N Engl J Med
1992;327:1769-73.
[ Return
to Text ] [ Abstract
]
9. Warshafsky S, Nowakowski J, Nadelman RB, Kamer RS, Peterson SJ, Wormser GP.
Efficacy of antibiotic prophylaxis for prevention of Lyme disease. J Gen Intern
Med 1996;11:329-33.
[ Return
to Text ]
10. Magid D, Schwartz B, Craft J, Schwartz JS. Prevention of Lyme disease after
tick bites: a cost-effectiveness analysis. N Engl J Med 1992;327:534-41.
[ Return
to Text ] [ Abstract
]
11. Schroeter AL, Turner RH, Lucas JB, Brown WJ. Therapy for incu-bating
syphilis: effectiveness of gonorrhea treatment. JAMA 1971;218:711-3.
[ Return
to Text ]
12. Takafuji ET, Kirkpatrick JW, Miller RN, et al. An efficacy trial of
doxycycline chemoprophylaxis against leptospirosis. N Engl J Med
1984;310:497-500.
[ Return
to Text ] [ Abstract
]
13. Falco RC, McKenna DF, Daniels TJ, et al. Temporal relation between Ixodes
scapularis abundance and risk for Lyme disease associated with erythema
migrans. Am J Epidemiol 1999;149:771-6.
[ Return
to Text ]
14. Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme
disease-endemic area. Am J Epidemiol 1996;143:187-92.
[ Return
to Text ]
15. Nadelman RB, Pavia CS, Magnarelli LA, Wormser GP. Isolation of Borrelia
burgdorferi from the blood of seven patients with Lyme disease. Am J Med
1990;88:21-6.
[ Return
to Text ]
16. Recommendations for test performance and interpretation from the Second
National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal
Wkly Rep 1995;44:590-1.
[ Return
to Text ]
17. O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials.
Biometrics 1979;35:549-56.
[ Return
to Text ]
18. Hennekens CH, Buring JE. Description and analysis of data. In: Epidemiology
in medicine. 1st ed. Boston: Little, Brown, 1987:254-7.
[ Return
to Text ]
19. Nadelman RB, Nowakowski J, Forseter G, et al. The clinical spectrum of
early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am
J Med 1996;100:502-8.
[ Return
to Text ]
20. des Vignes F, Piesman J, Heffernan R, Schulze TL, Stafford KC III, Fish D.
Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia
phagocytophila by Ixodes scapularis nymphs. J Infect Dis 2001;183:773-8.
[ Return
to Text ]
21. Sood SK, Salzman MB, Johnson BJB, et al. Duration of tick attachment as a
predictor of the risk of Lyme disease in an area in which Lyme disease is
endemic. J Infect Dis 1997;175:996-9.
[ Return
to Text ]
22. Falco RC, Fish D, D'Amico V. Accuracy of tick identification in a Lyme
disease endemic area. JAMA 1998;280:602-3.
[ Return
to Text ]
Appendix
Other investigators in the Tick Bite Study Group are as follows:
Susan Bittker, M.S., Denise Cooper, B.S., Diane Holmgren, R.N., and Charles
Pavia, Ph.D., from the Department of Medicine, Division of Infectious Diseases,
and Ira Schwartz, Ph.D., from the Department of Biochemistry and Molecular
Biology, New York Medical College, Valhalla.
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.