Notice:
Because of their potential importance in the treatment of Lyme disease, these articles
are being published early (on June 12, 2001). The final versions will appear in
the July 12 issue of the Journal.
![]()
Two Controlled Trials of
Antibiotic Treatment in Patients with Persistent Symptoms and a History of Lyme
Disease
Mark S. Klempner, M.D., Linden T. Hu, M.D., Janine Evans,
M.D., Christopher H. Schmid, Ph.D., Gary M. Johnson, Richard P. Trevino, B.S.,
DeLona Norton, M.P.H., Lois Levy, M.S.W., Diane Wall, R.N., John McCall, Mark
Kosinski, M.A., and Arthur Weinstein, M.D.
ABSTRACT
Background It is
controversial whether prolonged antibiotic treatment is effective for patients
in whom symptoms persist after the recommended antibiotic treatment for acute
Lyme disease.
Methods We conducted
two randomized trials: one in 78 patients who were seropositive for IgG
antibodies to Borrelia burgdorferi
at the time of enrollment and the other in 51 patients who were seronegative.
The patients received either intravenous ceftriaxone, 2 g daily for 30 days,
followed by oral doxycycline, 200 mg daily for 60 days, or matching intravenous
and oral placebos. Each patient had well-documented, previously treated Lyme
disease but had persistent musculoskeletal pain, neurocognitive symptoms, or
dysesthesia, often associated with fatigue. The primary outcome measures were
improvement on the physical- and mental-health–component summary scales of the
Medical Outcomes Study 36-item Short-Form General Health Survey (SF-36) — a
scale measuring the health-related quality of life — on day 180 of the study.
Results After a
planned interim analysis, the data and safety monitoring board recommended that
the studies be discontinued because data from the first 107 patients indicated
that it was highly unlikely that a significant difference in treatment efficacy
between the groups would be observed with the planned full enrollment of 260
patients. Base-line assessments documented severe impairment in the patients'
health-related quality of life. In intention-to-treat analyses, there were no
significant differences in the outcomes with prolonged antibiotic treatment as
compared with placebo. Among the seropositive patients who were treated with
antibiotics, there was improvement in the score on the physical-component
summary scale of the SF-36, the mental-component summary scale, or both in 37
percent, no change in 29 percent, and worsening in 34 percent; among
seropositive patients receiving placebo, there was improvement in 40 percent,
no change in 26 percent, and worsening in 34 percent (P=0.96 for the comparison
between treatment groups). The results were similar for the seronegative
patients.
Conclusions There is
considerable impairment of health-related quality of life among patients with
persistent symptoms despite previous antibiotic treatment for acute Lyme
disease. However, in these two trials, treatment with intravenous and oral
antibiotics for 90 days did not improve symptoms more than placebo.
Notice: Because of their
potential importance in the treatment of Lyme disease, these articles are being
published early (on June 12, 2001). The final versions will appear in the July
12 issue of the Journal.
Antibiotic treatment is highly effective for the acute and late
septic manifestations of Lyme disease, which is caused by the tick-borne
bacterium Borrelia burgdorferi.1
However, some patients have persistent fatigue, myalgias, arthralgias without
arthritis, dysesthesias or paresthesias, or mood and memory disturbances after
the standard courses of antibiotics.2
3
Persistent symptoms have been reported both in patients who are seropositive
for antibodies against B. burgdorferi
and in patients who are seronegative. Although the cause of persistent symptoms
has not been determined, their temporal association with B. burgdorferi infection has led some
physicians to treat patients with prolonged courses of antibiotics. Case
reports and uncontrolled trials describe success with prolonged antibiotic
therapy, often with a recurrence of the symptoms after the discontinuation of
therapy.4
In view of the substantial morbidity and even death5
associated with prolonged parenteral antibiotic treatment of Lyme disease, it
is important to determine the efficacy of such therapy. We report results from
randomized, placebo-controlled, double-blind trials of antibiotic therapy in
seropositive and seronegative patients who had chronic symptoms after treatment
for Lyme disease.
Methods
Patients
Patients were recruited by means of advertisements and referrals
from physicians. Between July 24, 1997, and November 14, 2000, eligible
patients were enrolled in two double-blind, placebo-controlled trials, each
conducted at three sites. Patients with a positive Western blot for IgG
antibodies against B. burgdorferi
antigens6
were enrolled in a study of seropositive patients, and patients who were
seronegative were enrolled in a separate study. Seronegative patients were
required to have documentation of an erythema migrans skin lesion provided by
an experienced physician. We initially planned to enroll 260 patients in the
studies (194 seropositive and 66 seronegative patients). Patients were eligible
if they were at least 18 years old, had a history of acute Lyme disease
acquired in the United States, and had at least one of the following: a history
of single or multiple erythema migrans skin lesions, early neurologic or
cardiac symptoms attributed to Lyme disease, radiculoneuropathy, or Lyme
arthritis. Documentation by a physician of previous treatment of acute Lyme
disease with a recommended antibiotic regimen was also required. At the time of
enrollment, all patients had one or more of the following symptoms that
interfered with their functioning: widespread musculoskeletal pain, cognitive
impairment, radicular pain, paresthesias, or dysesthesias. Profound fatigue
often accompanied one or more of these symptoms. The chronic symptoms had to
have begun within 6 months after the initial infection with B. burgdorferi and had to have persisted
for at least 6 months but less than 12 years.
Patients were excluded if they had hypersensitivity to the study
medications, had previously received parenteral antibiotic therapy for 60 days
or more for their current symptoms, had active inflammatory synovitis, had a
coexisting condition that could have accounted for their symptoms, or were
unable to discontinue medications that could interfere with the evaluation of
their response to the treatment regimen (e.g., narcotic analgesics or
prednisone in a dose of 10 mg per day or more). Patients with a positive
polymerase-chain-reaction (PCR) test for B.
burgdorferi DNA in plasma or cerebrospinal fluid at base line were
also excluded.
Study Protocols
The study protocols were approved by the institutional review
boards for human investigations at the participating centers, and each patient
gave written informed consent. A data and safety monitoring board planned an
interim analysis after at least 110 subjects had been enrolled. Patients were
randomly assigned in a 1:1 ratio to receive either antibiotics or placebo. The
antibiotic treatment regimen consisted of intravenous ceftriaxone for 30
consecutive days (2 g per day), followed by oral doxycycline for 60 consecutive
days (100 mg twice daily). The patients in the placebo group received an
intravenous dextrose solution that was the same color as the ceftriaxone
solution and oral placebo capsules identical in appearance to the doxycycline,
both for the same lengths of time as the antibiotics were administered.
Compliance and safety were monitored by home visits by study nurses every other
day during the intravenous-treatment phase and twice (on days 45 and 75) during
the oral-treatment phase.
Clinical and laboratory evaluations were performed at screening,
at base line, and on days 3, 5, 13, 21, 30, 45, 75, 90, and 180. The base-line
evaluation included a complete medical history taking, a detailed physical
examination, neuropsychological testing, a lumbar puncture, and sampling of
peripheral blood.
Clinical Response
The primary outcome was an improvement in the patients'
health-related quality of life, which was measured by means of the Medical
Outcomes Study (MOS) 36-item Short-Form General Health Survey (SF-36).7
8
Additional measures of the health-related quality of life included the MOS
scales for pain, cognition, and role functioning (the ability to participate in
usual daily activities)9
10
11
and a modified version of the Fibromyalgia Impact Questionnaire (FIQ).12
13
Each of the instruments used to measure the health-related quality of life was
administered to study participants at base line and at 30, 90, and 180 days.
The SF-36 includes eight multiple-item scales that measure physical
functioning, physical limitations on usual role-related activities, bodily
pain, general health perceptions, vitality, social functioning, emotional
limitations on usual role-related activities, and mental health. These scales
provide the basis for calculating the summary scores on the physical component
and the mental component of the SF-36.7
8
The scores range from 0 (worst) to 100 (best). For each of the scales, the mean
(±SD) score for members of the general population of the United States without
chronic conditions was considered to be 50±10.
To assess changes in the health-related quality of life over time,
a change score for each SF-36 summary scale was calculated by subtracting the
base-line score from the scores at 30, 90, and 180 days. With the use of 2 SE
as the criterion,8
14
individual patients were classified into three categories of change: those
whose follow-up scores did not change more than would be expected by chance
(unchanged group); those whose follow-up scores improved by more than 2 SE
(improved group); and those whose follow-up scores declined by more than 2 SE
(worse group). According to previous studies, a change of 2 SE is 6.5 points on
the SF-36 physical-health summary scale and 7.9 points on the SF-36
mental-health summary scale.8
14
The overall change in health status was calculated for each patient. Patients
were categorized as having improved health status if they had better scores on
both scales or had a better score on one scale and a score on the other scale
that was not worse. Patients were categorized as having worsened health status
if they had worse scores on one or both of the scales and as having the same
health status if their scores on both the physical- and mental-component scales
were unchanged.
Three additional multiple-item scales from the MOS were used as
secondary measures of cognitive functioning, pain, and functioning in
role-related activities.9
10
11
15
Clinical response was also measured with the use of the modified version of the
FIQ, for which the scores range from 0 (best) to 110 (worst). This version of
the FIQ has been validated for patients who have persistent symptoms after
treatment for Lyme disease.13
In a control population without chronic illness, the mean total score on the
modified FIQ was 14.0, whereas the mean for patients with chronic Lyme disease
after treatment was 50.2.13
Previous studies have indicted that a change of 16.0 points is clinically
meaningful.16
17
18
19
Laboratory Studies
Western blotting for IgG antibodies against B. burgdorferi antigens was performed with
the IgG MarBlot (MarDx Diagnostics, Carlsbad, Calif.) according to the
manufacturer's instructions.6
The intrathecal production of antibodies against B. burgdorferi was measured as previously described.20
Base-line specimens of cerebrospinal fluid and plasma specimens obtained at
base line and on days 3, 5, 21, and 45 were tested by PCR for the presence of B. burgdorferi DNA, as previously
described.21
All samples of cerebrospinal fluid were cultured in Barbour–Stoenner–Kelly II
medium to detect B. burgdorferi
and were monitored by dark-field microscopy for six weeks.22
Some blood samples were cultured for B.
burgdorferi in hypertonic medium.23
Adverse Events
Adverse events and changes in laboratory values were evaluated and
graded with the use of scales derived from the Common Toxicity Criteria of the
National Cancer Institute.
Compliance
Compliance with the regimen of medications was assessed by counts
of both the number of doses of the intravenous medication the patients received
and the pills they had remaining. The actual pill counts were compared with the
number of pills that the patient should have had remaining at the time of the
home visit by a nurse on day 75.
Statistical Analysis
The primary analysis was an intention-to-treat analysis of all the
patients enrolled in the studies. The primary clinical end point was the
proportion of patients whose condition was categorized as improved, unchanged,
and worse on the basis of the summary scores for the mental and physical
components of the SF-36 at 180 days. Patients who withdrew from the study were
categorized as having worsened health status on both of these scales.
The study of seropositive patients was designed to have a power of
90 percent, with a 5 percent level of significance in a two-sided test and a
sample size of 194, to detect a difference of 25 percent between the antibiotic
group and the placebo group in the proportion of patients with improved health
status.24
With the enrollment of 66 patients, the study of seronegative patients would
have 80 percent power, with a 5 percent level of significance in a two-sided
test, to detect a 35 percent difference between the antibiotic group and the
placebo group.24
In the interim analysis of the primary outcome, the
O'Brien–Fleming adjustment for multiple testing and the B-value stochastic
curtailment method were used.25
The primary outcome for each trial was analyzed by the chi-square test with
individual type I error rates of 0.05. The efficacy of the treatment in each
trial was estimated as the difference in risk (with 95 percent confidence
interval). Secondary outcomes included improvement or worsening as measured by
the SF-36 at 30 and 90 days, the total FIQ score, and the MOS pain, cognition,
and role-functioning scores at 30, 90, and 180 days.
Results
Characteristics of the Patients
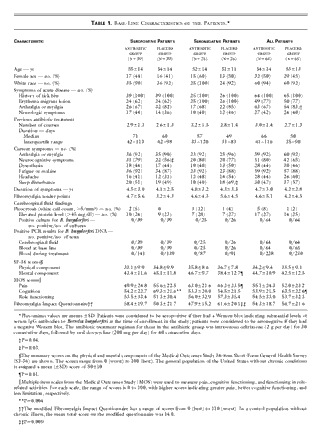
A total of 129 patients were enrolled in the studies (78
seropositive and 51 seronegative). The base-line characteristics of the
patients are shown in Table
1. A total of 42 (33 percent) of the patients had previously received
intravenous antibiotic treatment for a mean (±SD) of 30±12 days. All other
previous treatment consisted of oral antibiotics. There were no significant
differences between the seropositive patients and the seronegative patients other
than the presence or absence of serum antibodies to B. burgdorferi. All cultures of cerebrospinal fluid in
standard Barbour–Stoenner–Kelly II medium and of blood in hypertonic medium
were negative for B. burgdorferi.
B. burgdorferi DNA was not
detected in any base-line sample of cerebrospinal fluid or blood, nor was it
detected in any of the blood samples obtained during the treatment phase (on
days 3, 5, 21, and 45). Given a cutoff of 1.2 for the ratio of antibody against
B. burgdorferi in cerebrospinal fluid
to antibody in serum, the intrathecal production of antibody was detected in
eight patients (five in the combined antibiotic groups and three in the
combined placebo groups).
Treatment was discontinued in 14 patients (8 seropositive and 6
seronegative). The reasons for the discontinuation of treatment were similar
across the groups of patients. An adverse event was the reason for the
discontinuation of treatment in three of the patients in the combined
antibiotic groups (5 percent) and three of those in the combined placebo groups
(5 percent). Treatment was discontinued in the other eight patients for reasons
other than an adverse event.
In the combined population of both studies, the mean base-line
summary score for the physical component of the SF-36 was approximately 1.5 SD
below that of age-matched members of the general population of the United
States who did not have a chronic illness, and the mean base-line summary score
for the mental component was approximately 0.5 SD below that of such a control
group. Similarly, the base-line FIQ scores were markedly abnormal.
Compliance
All patients who completed 180 days in one of the trials took at
least 75 percent of the prescribed medications. There were no differences
between the treatment groups in either the seropositive or the seronegative
study in patients' compliance with medication.
Efficacy
The interim analysis was performed with the use of data on 107
patients who had completed 180 days in one of the trials. This analysis
indicated that there was a 1.4 percent chance in the seropositive study and a
4.0 percent chance in the seronegative study that a significant difference in
the efficacy of treatment between the antibiotic group and the placebo group
would be observed when the full projected enrollment was reached. An analysis
that included the patients in both studies indicated that there was a 3.9
percent chance of observing a significant difference at full enrollment.
Because of the lack of efficacy of the antibiotic treatment at the time of the
interim analysis, the data and safety monitoring board recommended
discontinuation of the active treatment of enrolled patients and of further
enrollment in the treatment phase of the studies.
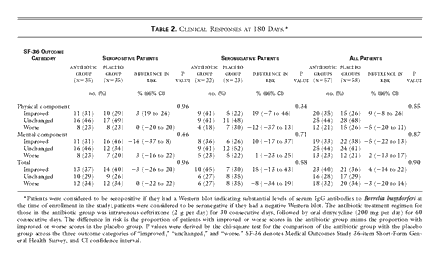
The responses of the 115 patients who were enrolled in the studies
at least 180 days before enrollment was stopped are shown in Table
2 and Figure
1. Intention-to-treat analyses at 30, 90, and 180 days showed no
significant differences in the measures of the health-related quality of life
between the patients in the antibiotic groups and those in the placebo groups
in the seropositive study, the seronegative study, or both studies combined.
In the combined study populations, changes in the score on the
modified FIQ at 180 days also revealed no significant differences between the
antibiotic groups and the placebo groups. We defined a decrease of 25 percent
from the base-line score on the modified FIQ as indicative of clinical
improvement and an increase of 25 percent as indicative of clinical worsening.
Given these definitions, 28 of the 51 patients in the combined antibiotic
groups (55 percent) had improved health status as measured by the FIQ at 180
days, as compared with 22 of the 53 patients in the combined placebo groups (42
percent) (P=0.17). Conversely, 14 percent of the patients in the antibiotic
groups (7 of 51) had worsened health status as measured by the FIQ at 180 days,
as compared with 19 percent (10 of 53) in the placebo groups (P=0.48). Separate
comparisons of the FIQ scores in the antibiotic group and the placebo group of
the seropositive study and of those in the two treatment groups in the
seronegative study found no statistically significant differences according to
treatment.
Adverse Events
At least one study-related adverse event occurred in 16 of the 64
patients in the combined antibiotic groups (25 percent) and 11 of the 65
patients in the combined placebo groups (17 percent). Most of the adverse
events were minor and resolved without intervention or sequelae. However, the
two patients in whom a study-related serious adverse event occurred were both
in the antibiotic group. During intravenous treatment, one had a
life-threatening pulmonary embolism and the other had fever, anemia, and
gastrointestinal bleeding. Although the overall rate of adverse events was not
significantly different in the two treatment groups, rash, diarrhea, and vaginal
pruritus occurred more frequently among the patients in the antibiotic groups
(9 of 64 patients) than among those in the placebo groups (2 of 65 patients).
There were no infections associated with the intravenous catheter, and there
were no deaths.
Discussion
The primary goals of these studies in patients with symptoms that
persist after recommended antibiotic treatment for Lyme disease were to
characterize the impairment in health-related quality of life among such
patients and to determine the efficacy of prolonged treatment with antibiotics.
The effect of chronic Lyme disease on the health-related quality of life was
substantial in both seropositive and seronegative patients. The deficits in
physical health status as measured by the SF-36 were equivalent to those
observed in patients with congestive heart failure or osteoarthritis and were
more than 0.5 SD greater than the impairment observed in patients with type 2
diabetes or a recent myocardial infarction.7
8
Chronic pain was an important contributor to the impairment of physical health
and was similar to that reported by patients with osteoarthritis.7
8
The impairment of mental health status was similar to that observed in patients
with subthreshold lifetime depression (a depressive disorder that does not meet
all of the criteria for major depression of the Diagnostic and Statistical Manual of Mental Disorders, third
edition).7
8
26
The study patients also had some impairment in cognitive functioning. Their
base-line FIQ scores reflected the impairments in health status that were
evident in the SF-36 scores and were similar to those in previous studies of
patients with fibromyalgia or those with chronic Lyme disease after treatment.8
9
10
11
12
13
The administration of placebo and treatment with a regimen of
parenteral ceftriaxone for 30 days, followed by oral doxycycline for 60 days,
had similar effects on the patients' health-related quality of life. This
antibiotic-treatment regimen was selected because of the in vitro and in vivo
activity of both of these antibiotics against B.
burgdorferi and because they are effective for the treatment of
neuroborreliosis.1
Experience with other chronic infectious diseases caused by persistent bacteria
(e.g., syphilis, tuberculosis, and helicobacter infection) suggests that it is
unlikely that more prolonged antibiotic therapy or a different combination of
antibiotics would result in greater improvement than was observed in this
study.
Although we used both conventional and hypertonic culture mediums
to isolate B. burgdorferi in
base-line samples of cerebrospinal fluid and blood and used PCR to detect B. burgdorferi DNA in base-line samples of
blood and cerebrospinal fluid as well as samples of blood collected during
treatment, we did not find evidence of persistent infection with B. burgdorferi in these patients. There
was no evidence of B. burgdorferi in
a total of more than 700 different blood and cerebrospinal fluid samples from
the 129 patients in these studies.
The placebo groups in these studies provide a view of the natural
history of symptoms among patients with chronic Lyme disease after treatment.
During the six-month evaluation period, we observed improvement in health
status in 36 percent of patients, worsening health status in 39 percent, and no
significant change in 25 percent. A similar relation between the administration
of placebo and health-related quality of life has been reported previously
among patients with other rheumatologic diseases that do not appear to be
related to persistent infection. For example, a randomized, placebo-controlled
trial in patients with mild-to-moderate rheumatoid arthritis found an improved
clinical response after 48 weeks of placebo treatment in 39 to 41 percent of
patients, as compared with 54 to 56 percent of patients treated with
minocycline.27
In summary, patients with chronic musculoskeletal pain,
neurocognitive symptoms, or both that persist after antibiotic treatment for
well-documented Lyme disease have considerable impairment in their
health-related quality of life. The patients enrolled in these studies did not
have evidence of persistent infection by B.
burgdorferi, as judged on the basis of the available microbiologic
and molecular methods of detection. There were no significant differences
between clinical responses of patients who received intravenous and oral
antibiotics for 90 days and those of patients who received placebo.
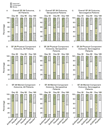
Figure 1. Change or Lack of Change from
Base Line in the Health-Related Quality of Life as Measured by the Medical
Outcomes Study 36-Item Short-Form General Health Study (SF-36). Results are shown for the overall outcome
(Panel A, all patients; Panel D, seropositive patients; Panel G, seronegative
patients); the physical component (Panel B, all patients; Panel E,
seropositive patients; Panel H, seronegative patients); and the mental
component (Panel C, all patients; Panel F, seropositive patients; Panel I,
seronegative patients). The scores of patients in the antibiotic group are
compared with those of patients in the placebo group at the end of
intravenous treatment (day 30), at the end of oral treatment (day 90), and 3
months after the completion of treatment (day 180). Statistical tests
comparing the scores in the antibiotic group with those in the placebo group
showed no statistically significant difference at any of the time points.
|
Table 1. Base-Line Characteristics of the
Patients.
|
Table 2. Clinical Responses at 180 Days.
|
Source Information
From New England Medical Center and Tufts University School of Medicine,
Boston (M.S.K., L.T.H., C.H.S., G.M.J., R.P. T., J.M.); Yale–New Haven
Hospital, New Haven, Conn. (J.E., D.W.), New York Medical College, Valhalla
(D.N., L.L., A.W.); and Quality Metric, Lincoln, R.I. (M.K.). Address reprint
requests to Dr. Klempner at the Department of Medicine, Boston University
School of Medicine, 715 Albany St., Boston, MA 02118, or at [log in to unmask].
Supported by grants (N01-AI-65308 and M01 RR000054) from the
National Institutes of Health. Roche provided the ceftriaxone for the study,
and Pfizer provided the doxycycline.
We are indebted to Philip J. Baker, Ph.D., Steven P. Heyse, M.D.,
Marilyn Tuttleman, M.S., Dennis Dixon, Ph.D., and Mark Van Raden, Ph.D., of the
National Institute of Allergy and Infectious Diseases Lyme Disease Program; to
Barbara E. Murray, M.D., Robert Edelman, M.D., Frank G. Miller, Ph.D., Donald
Rosenstein, M.D., and Barbara Tilley, Ph.D., of the data and safety monitoring
board; to John E. Edwards, Jr., M.D., Carl Brenner, J. Stephen Dumler, M.D.,
Fred A. Gill, M.D., Phyllis Mervine, Justin Radolf, M.D., and Gregory Owens,
Ph.D., of the Scientific Advisory Committee; and to the following persons who
provided helpful services: Rich Noring, Bilaal McCloud, Bo Lin, Ph.D., Richard
Kaplan, Ph.D., David Weld, Roger D'Entremont, Kitty Mullen, Chris Covington,
Vincent DiPaola, Paul Drouilhet, David Fletcher, Jerrold Gold, Thomas Hirsch,
Clyde Kessel, Keith Krewson, Laura Lattanzio, Rebecca Leland, Shawn Lyden,
William Macleod, Roy Matthews, Peter Morton, Dave Murray, Jim Platz, John
Sample, Elliott Schiffman, Michael Shabazian, Jason Stone, John Taylor,
Bradford Von Weise, Frank Weldon, Larry Camerlin, Raymond Partridge, M.D.,
Marianna Wilson, M.S., John Consoletti, Pharm.D., Cindy Mason, R.N., David
Thorpe, Pharm.D., Cindy Moore, R.N., June Novio, R.N., Eileen Regan, R.N., Andy
Dousa, Pharm.D., Don Morrison, Pharm.D., Laura Cavagna, Pharm.D., Linda
Griffith, Pharm.D., Sean Corbett, Pharm.D., Scott Reid, Pharm.D., Fran Bickert,
R.N., John Opolski, R.N., Karen Tooker, R.N., Mark Wolff, Ph.D., Phyllis Barr,
Ph.D., Anna Lornell, M.D., Phil Molloy, M.D., Karen Forschner, Dennis Hoak,
M.D., David N. Mesches, M.D., Tim Lepore, M.D., Robina Folland, Robert Lopez,
Bai Margolin, Crystal Sylvester, George Perides, Ph.D., Allen Steere, M.D.,
Ellen Jamieson, Jim Grayson, Timothy Brauns, Lilla Rogers, Jenn Finn, R.N.,
Pamela Norton, R.N., Karen Mazzotta, Anh Nguyen, M.D., Eric Logigian, M.D.,
William Brown, M.D., Anne Donohoe, Sandy Doveikis, Karen Fenicchia, R.N., Hua
Wang, R.N., Nancy Zajac, R.N., Jamie Evanowski, David Carlson, Jason Hoitt,
Carol Bauscher, L.P.N., Teryi Deshefy-Longhi, R.N., M.S., Carl Henn, Dee Dee
Moss, Glynnis Fisher, Janet Mattson, Rosemary Hammil, Beth Horrigan, William
Blackwelder, M.D., Frank Cavileri, M.D., John Nowakowski, M.D., Morris Dannon,
M.D., Brij Mohan Singh Ahluwalia, M.D., Gary Wormser, M.D., Rhea Dornbush,
Ph.D., Catherine Crea, Maria Aguero-Rosenfeld, M.D., Alan Gerber, M.D., Michael
Tenner, M.D., Maureen Grix, Ph.D., Lois Zentmaier, Denise Cooper, Susan
Bittker, Sheila Hughes, R.N., Junius Edlow, Tim McGarty, Jeffrey Bandola, M.D.,
Andrew Artenstein, M.D., Nancy Clark, R.N., Jerry Fingerut, M.D., Robert
Schoen, M.D., Donna D'Euginio, R.N., Linda Bockenstedt, M.D., Alexia
Bellperron, Brad Herskowitz, M.D., George Rickerson, M.D., LeBraun Paige, M.D.,
Volker Knappertz, M.D., Kimberly Stoddard, Ph.D., Ann Sweeney, R.N., Michael
Westerveldt, Ph.D., Maureen Tacey, Thomas Rush, M.D., Jill Auerbach, Betty Gross,
Manuel DaSilva, M.D., Arthur Rabson, M.D., Brian Fallon, M.D., Linda Tanner,
Richard Horowitz, M.D., Edmond Yunis, M.D., Paul Alexander, R.N., and Ann Marie
McClean, R.N.
References
1. Wormser GP, Nadelman RB, Dattwyler RJ, et al. Practice guidelines
for the treatment of Lyme disease. Clin Infect Dis 2000;31:Suppl 1:1-14.
[ Return
to Text ]
2. Asch ES, Bujak DI, Weiss M, Peterson MGE, Weinstein A. Lyme disease: an
infectious and post infectious syndrome. J Rheumatol 1994;21:454-61.
[ Return
to Text ]
3. Bujak DI, Weinstein A, Dornbush RL. Clinical and neurocognitive features of
the post Lyme syndrome. J Rheumatol 1996;23:1392-7.
[ Return
to Text ]
4. Donta ST. Tetracycline therapy for chronic Lyme disease. Clin Infect Dis
1997;25:Suppl 1:S52-S56.
[ Return
to Text ]
5. Patel R, Grogg KL, Edwards WD, Wright AJ, Schwenk NM. Death from
inappropriate therapy for Lyme disease. Clin Infect Dis 2000;31:1107-9.
[ Return
to Text ]
6. Recommendations for test performance and interpretation from the Second
National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal
Wkly Rep 1995;44:590-1.
[ Return
to Text ]
7. Ware JE Jr. SF-36 Health Survey: manual and interpretation guide. Boston:
Health Institute, New England Medical Center, 1993.
[ Return
to Text ]
8. Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary
Scales: a user's manual. Boston: Health Assessment Lab, 1994.
[ Return
to Text ]
9. Stewart AL, Ware JE Jr, Sherbourne CD, Wells KB. Psychological
distress/well-being and cognitive functioning measures. In: Stewart AL, Ware JE
Jr, eds. Measuring functioning and well-being: the Medical Outcomes Study
approach. Durham, N.C.: Duke University Press, 1992:102-42.
[ Return
to Text ]
10. Sherbourne CD, Stewart AL, Wells KB. Role functioning measures. In: Stewart
AL, Ware JE Jr, eds. Measuring functioning and well-being: the Medical Outcomes
Study approach. Durham, N.C.: Duke University Press, 1992:205-19.
[ Return
to Text ]
11. Sherbourne CD. Pain measures. In: Stewart AL, Ware JE Jr, eds. Measuring
functioning and well-being: the Medical Outcomes Study approach. Durham, N.C.:
Duke University Press, 1992:220-34.
[ Return
to Text ]
12. Burckhardt CS, Clark SR, Bennett RM. The Fibromyalgia Impact Questionnaire:
development and validation. J Rheumatol 1991;18:728-33.
[ Return
to Text ]
13. Fallon J, Bujak DI, Guardino S, Weinstein A. The Fibromyalgia Impact
Questionnaire: a useful tool in evaluating patients with post-Lyme disease
syndrome. Arthritis Care Res 1999;12:42-7.
[ Return
to Text ]
14. Ware JE Jr, Bayliss MS, Rogers WH, Kosinski M, Tarlov AR. Differences in
4-year health outcomes for elderly and poor, chronically ill patients treated
in HMO and fee-for-service systems: results from the Medical Outcomes Study.
JAMA 1996;276:1039-47.
[ Return
to Text ]
15. Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile:
development and final revision of a health status measure. Med Care
1981;19:787-805.
[ Return
to Text ]
16. Goldenberg D, Mayskiy M, Mossey C, Ruthazer R, Schmid C. A randomized,
double-blind crossover trial of fluoxetine and amitriptyline in the treatment
of fibromyalgia. Arthritis Rheum 1996;39:1852-9.
[ Return
to Text ]
17. Bennett RM, Clark SC, Walczyk J. A randomized, double-blind,
placebo-controlled study of growth hormone in the treatment of fibromyalgia. Am
J Med 1998;104:227-31.
[ Return
to Text ]
18. Bennett RM, Burckhardt CS, Clark SR, O'Reilly CA, Wiens AN, Campbell SM.
Group treatment of fibromyalgia: a 6 month outpatient program. J Rheumatol
1996;23:521-8.
[ Return
to Text ]
19. Dunkl PR, Taylor AG, McConnell GG, Alfano AP, Conaway MR. Responsiveness of
fibromyalgia clinical trial outcome measures. J Rheumatol 2000;27:2683-91.
[ Return
to Text ]
20. Steere AC, Berardi VP, Weeks KE, Logigian EL, Ackermann R. Evaluation of
the intrathecal antibody response to Borrelia burgdorferi as a diagnostic test
for Lyme neuroborreliosis. J Infect Dis 1990;161:1203-9.
[ Return
to Text ]
21. Nocton JJ, Bloom BJ, Rutledge BJ, et al. Detection of Borrelia burgdorferi
DNA by polymerase chain reaction in cerebrospinal fluid in Lyme
neuroborreliosis. J Infect Dis 1996;174:623-7.
[ Return
to Text ]
22. Klempner MS, Noring R, Rogers RA. Invasion of human skin fibroblasts by the
Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis 1993;167:1074-81.
[ Return
to Text ]
23. Phillips SE, Mattman LH, Hulinska D, Moayad H. A proposal for the reliable
culture of Borrelia burgdorferi from patients with chronic Lyme disease, even
from those previously aggressively treated. Infection 1998;26:364-7.
[ Return
to Text ]
24. Farrington CP, Manning G. Test statistics and sample size formulae for
comparative binomial trials with null hypothesis of non zero risk difference or
non-unity relative risk. Stat Med 1990;9:1447-54.
[ Return
to Text ]
25. Lan KKG, Wittes J. The B-value: a tool for monitoring data. Biometrics
1988;44:579-85.
[ Return
to Text ]
26. Wells KB, Burnam MA, Rogers WH, Hays R, Camp P. The course of depression in
adult outpatients: results from the Medical Outcomes Study. Arch Gen Psychiatry
1992;49:788-94.
[ Return
to Text ]
27. Tilley BC, Alarcon GS, Heyse SP, et al. Minocycline in rheumatoid
arthritis: a 48-week, double blind, placebo-controlled trial. Ann Intern Med
1995;122:81-9.
[ Return
to Text ]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.