Table of Contents
HIV Transmission
Intervention
Strategies
Biological
HIV Prevention Strategies: Reducing Infectiousness, Susceptibility, and the
Efficiency of Transmission
Antiretroviral
Therapy and HIV Transmission
Conclusion
References
Appendix:
Talking About Safer Sex With Your Patients
HIV Transmission
Epidemiology of HIV Transmission
HIV is spread from human to human by 3
routes[1]:
- blood
transmission (contaminated transfusions, needle sharing during drug use,
needle-stick injuries)
- vertical
transmission (mother to offspring during parturition or breastfeeding)
- sexual
transmission
Sexual transmission of HIV accounts for
more than 75% of infections worldwide.
The probability of transmission of HIV by different sexual routes
per episode of intercourse is summarized in Figure 1, using data generated by
epidemiologists and mathematical modeling.[1] Transmission of HIV from men to their
partners is more efficient than from women to men.[2,3] Transmission of HIV through anal
intercourse is more efficient than other sexual behaviors. In addition,
transmission per episode of intercourse may well be affected by the stage of
disease of the infected subject or innate or acquired immunity of the exposed
person as discussed below. Oral sex between women (cunnilingus) appears to confer
almost no risk, and fellatio appears to have limited risk relative to sexual
intercourse. However, HIV acquisition among gay men has been reported as a
result of fellatio alone.[4] Among 122 individuals with primary HIV infection, Dillon
and coworkers[5] attributed 6.6% of cases to oral sex. In addition, simian
immunodeficiency virus (SIV) has been transmitted to macaques through oral
inoculation using similar concentrations of virus as those required for vaginal
infection.[6,7]
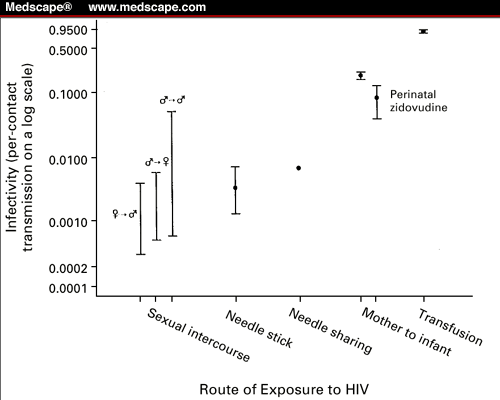
Figure 1. Per-contact probability of HIV
transmission. The infectivity ranges for sexual contact are derived from a
comprehensive review of the literature (lower and upper bounds are from
modeling per-contact transmission in different study populations with different
modeling techniques). Each infectivity estimate for the other routes of
infection originates from one representative study. The routes of infection are
as follows: sexual intercourse, with ![]() indicating female-to-male
transmission,
indicating female-to-male
transmission, ![]() indicating male-to-female
transmission, and
indicating male-to-female
transmission, and ![]() indicating male-to-male transmission;
needle stick; needle sharing; transmission from mother to infant with and
without perinatal zidovudine treatment; and transfusion.
indicating male-to-male transmission;
needle stick; needle sharing; transmission from mother to infant with and
without perinatal zidovudine treatment; and transfusion.
Royce RA, Sena A, Cates W Jr, Cohen MS. Current concepts: sexual transmission
of HIV. N Engl J Med. 1997;336:1072-1078. Copyright 1997. Massachusetts Medical
Society. All rights reserved.
The spread of HIV can be assessed at a population level as well.
Anderson and May[8] have described the risk of secondary (new) cases of HIV as
Ro, where Ro = beta x C x delta, with beta representing the efficiency of
transmission, C the number of sexual partners, and delta the duration of
infectiousness of the index case. When Ro exceeds 1, new, secondary cases of
HIV occur, and the epidemic continues. Successful prevention strategies must
reduce Ro to less than 1 and include lowering the rate of partner change,
reducing the efficiency of transmission, and shortening the duration of
infectiousness. This model offers an excellent conceptual framework to approach
HIV prevention and a tool to examine the success of interventions.
Biology of HIV Transmission
The efficiency of transmission of HIV
represents a biological event; transmission either does or does not occur. HIV
transmission must depend on the infectiousness of the index case (reviewed in
the paper by Vernazza and coworkers[9]) and the susceptibility of the exposed host (reviewed in
the paper by Buchacz and colleagues[10]). A schematic representing the transmission of HIV from a
male to a female partner is provided in Figure 2.
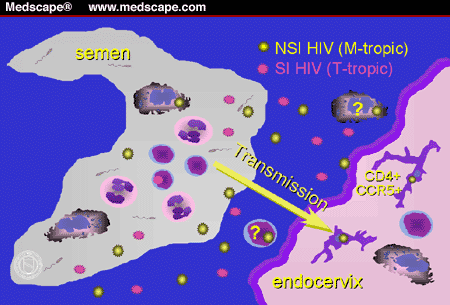
Figure 2. Male-to-female transmission of HIV.
Infectiousness of HIV. HIV can be recovered from seminal cells
(CD4+ macrophages and lymphocytes) and seminal plasma. Detection of HIV in
seminal plasma by RNA polymerase chain reaction amplification techniques has
been used as a surrogate for the concentration of HIV in semen, although
procedures to eliminate inhibition of amplification must be used.[11] When the concentration of HIV in seminal
plasma exceeds 10,000 copies/mL, HIV can usually be grown in seminal cells.[12] However, it is unclear whether HIV is
transmitted from seminal cells or seminal plasma; cell-free virions in seminal
plasma may be defective (and unfit).
The recovery of HIV from cervical mucus and cervicovaginal lavage
fluid is similar to semen, although it has only recently been possible to
quantify the copy number.[13,14] HIV can be recovered from cervicovaginal
lavage fluid cells when the concentration exceeds 10,000 copies/mL.[15]
Several lines of evidence suggest that the concentration of HIV in
genital secretions can be correlated with risk of sexual transmission. First,
there is overwhelming evidence that the concentration of HIV in an infected
mother's blood determines the risk of vertical transmission,[16-18] although one must realize that the
biologic mechanisms of vertical and sexual transmission are different. Second,
a correlation has been demonstrated between increased concentrations of HIV in
blood and enhanced transmission by all routes.[18-21] In a remarkable study in Uganda, Quinn
and coworkers[22] demonstrated that HIV transmission in HIV serodiscordant
couples could be correlated directly with the blood plasma HIV RNA level in the
infected subject. No HIV transmission was observed when blood plasma HIV was
less than 1500 copies/mL. In a similar study among serodiscordant couples in
Rakai, Uganda, Gray and colleagues[23] calculated that when the blood plasma viral load was less
than 3500 copies/mL, the transmission probability was 0.0001 (1 per 10,000
episodes of intercourse). When blood viral burden was greater than 50,000
copies/mL, the transmission probability was calculated to be 0.0051 (5.1 per
1000 episodes of intercourse). It should be noted the concentration of HIV in
blood can be directly (but imperfectly) correlated with the concentration of
HIV in semen[11,12,24] and female genital secretions.[15]
Third, the concentration of HIV in genital tract secretions from
males and females is increased at times when enhanced transmission is
suspected, such as in primary infection or in later stages of HIV disease,[11,12,15,24,25] and in patients with classic sexually
transmitted diseases (STDs) (reviewed in the paper by Fleming and Wasserheit[26]). However, recent data suggest that the
concentration of HIV in semen during primary infection may not be as high as
that in blood[27] and can overlap the concentrations
observed in seminal plasma throughout the disease.[28] These data would indicate that primary
infection is associated with a higher risk of transmission for reasons other
than viral burden in the genital tract or that the model suggesting that most
sexual HIV transmission may result from subjects with primary infection is
flawed.[29]
Empiric data on transmission and stage of disease are desperately
needed to inform public health policy.[30] Recent reports[31,32] indicate that individuals with
symptomatic primary HIV infection may infect sexual partners as early as 2 days
before the onset of the acute retroviral syndrome, or 10-18 days after
infection, suggesting that education targeting persons diagnosed with primary
HIV infection may be too late, because transmission may have already occurred.
Investigators at the University of North Carolina at Chapel Hill
have used HIV RNA in semen samples from several hundred men to estimate the
effects of semen viral burden on heterosexual transmission.[33,34] They concluded that when HIV RNA in
semen was low (<5000 copies/mL) transmission was unlikely to occur, at 1 per
10,000 episodes of intercourse. Conversely, when the concentration of HIV in
semen was high (eg, 1 million copies/mL) the probability of transmission rose
to 3 per 100 episodes of intercourse. Such high concentrations of HIV RNA have
been detected in semen specimens harvested from HIV-positive men in Africa
infected with STDs, including trichomonas and gonorrhea. Thus, the magnitude of
the AIDS epidemic in Africa likely reflects a variety of factors that
dramatically increase genital shedding of HIV.
Phenotypic requirements for HIV
transmission. HIV recovered from genital secretions is not homogeneous;
rather, it consists of a swarm of viral variants that can be separated into
discrete quasispecies. Virologic factors required for HIV transmission are only
poorly understood, but based on studies of isolates transmitted from infected
patients to their partners, it seems clear that some viral quasispecies in a
swarm appear to be favored.[35,36] In particular, homogeneous viral
isolates with envelope sequences that define the slow-growing,
macrophage-trophic, non-syncytium-inducing (NSI) phenotype are preferentially
transmitted.[37,38]
This observation implies that these variants overwhelm innate or
acquired resistance to infection and/or, more likely, that the genital mucosa
provide receptors, such as CD4 and CCR-5, that are more appropriate for this
viral phenotype than others.[39] Patterson and coworkers[40,41] have demonstrated availability of cells
with CD4 and CCR-5 receptors in the endocervix, where infection is likely
initiated. These receptors are subject to dynamic regulation by cytokines and
other inflammatory stimuli.[42]
A key question is whether HIV is transmitted by infected seminal
cells or by cell-free virus in the seminal plasma. SIV and SHIV (viral
constructs incorporating elements of both simian and human immunodeficiency
viruses) are more readily transmitted vaginally to macaques through cell-free
virus.[43]
In a recent study, DePasquale and colleagues carefully categorized the genotype
of HIV variants in the seminal plasma and the seminal cells of an infected
patient and that of HIV recovered from the blood of the patient's sexual
partner during his primary infection.[36] The results demonstrate that the
transmitted variant most likely originated in the seminal cells of the
infecting partner. Potent antiretroviral therapy that reduces HIV RNA in blood
plasma to below quantifiable levels is almost always effective at reducing HIV
RNA in seminal plasma to a similar extent.[9,44] However, the reservoir of HIV in seminal
cells may be difficult to eliminate even in the face of potent antiretroviral
therapy.[45,46]
HIV variants can be divided into subtypes or clades based on the
genotypic similarity in the HIV envelope region and in other genes as well.
Clade B is the dominant strain in the United States and Europe, whereas clades
A and C are dominant in sub-Saharan Africa, though all subtypes are
represented. Clades E and B predominate in Thailand[47] and some other parts of Asia. A variety
of clades and a recombinant B-C clade have been identified in the People's
Republic of China. Given the geographic differences in the magnitude of the HIV
epidemic, many investigators have tried to prove that different clades might be
transmitted with different efficiency. Stimulated by epidemiological evidence
that clade E is more infectious,[48] Soto-Ramirez and coworkers studied growth of different
clades of HIV in cervical epithelial cells and dendritic cells. They reported
increased growth of clade E HIV in these tissues.[49] However, these results could not be
reproduced by other investigators,[50,51] who also emphasized the technical difficulties
of this type of study.
Duerr and colleagues[52] presented evidence for clade-specific transmission from
men to women by comparing the efficiency of transmission of clade B HIV in 51
Italian couples (1.1 per 1000 episodes of intercourse) to clade E in 78 Thai
couples (2.4 per 1000 episodes of intercourse); however, these differences were
not significant. It seems probable that the actual concentration of HIV in the
genital tract is the most important determinant of transmission.
We[53] and others[54] have made observations related to clade C HIV that may be
relevant to transmission. This clade represents more than half of all HIV
infections on the planet and is predominant in hyperendemic regions such as
sub-Saharan Africa. Study of clade C variants from several different countries
has demonstrated that as patients develop more advanced disease, the virus
retains the NSI phenotype. This observation contrasts with evidence regarding
subtype B in North America and Europe, where a substantial proportion of
infected individuals have a shift in envelope phenotype from NSI to the
rapid-growing, lymphotropic, syncytia-inducing (SI) viral phenotype.[55-57] Retention of the NSI phenotype
throughout the disease course for most individuals with subtype C could also
promote sexual transmission of HIV through the efficient use of the CD4/CCR-5
receptors that are believed critical for sexual transmission (see below and
Figure 2). Recently, however, another group has reported a small number of
subtype C variants that appear to have the SI phenotype.[58]
Susceptibility to HIV Infection
Hereditary resistance to HIV. Susceptibility to HIV infection depends
on the lack of innate, hereditary resistance or acquired immunity. Recent
studies of hereditary resistance have helped to clarify this issue. Soon after
the discovery of HIV as the cause of AIDS, binding of the viral envelope to the
CD4 molecule on target cells was recognized, but it has been known for some
time that at least one additional receptor is also required for cell entry,
because CD4-expressing nonhuman cell lines are resistant to HIV infection.
Until recently, this receptor had escaped identification, but 2 important
second receptors, CCR-5 and CXCR-4, have now been described (reviewed in the
papers by Littman[39] and Hoffman and Doms[59]). The CCR-5 receptor is the natural
ligand for chemoattractants, including macrophage inflammatory protein 1 alpha
and beta and RANTES. The importance of the CCR-5 receptor was quickly
demonstrated when individuals who were homozygous for a nonfunctional CCR-5
mutation (a 32-base pair deletion) were found to be underrepresented in
HIV-1-infected populations[60] and overrepresented in uninfected exposed populations.[61] Cells from these individuals were shown
to be resistant to infection with NSI, macrophage-tropic HIV-1.[60] This CCR-5 mutation is observed in 1 of
100 whites[60] but is much less common among blacks and is associated
with a reduced risk of infection after exposure to HIV during intercourse, with
no apparent decrement in host defenses.[62]
These observations confirmed and explained the critical importance
of the NSI phenotype for sexual transmission, as suspected from studies cited
earlier that identified that patients with primary HIV infection typically have
a homogeneous NSI viral swarm, regardless of the route of transmission.
Innate and acquired resistance to HIV. Not every episode of intercourse has an
equal risk for HIV transmission. Some exposed, uninfected people do not have
the advantage of hereditary resistance, perhaps suggesting the contribution of
innate or acquired host defenses against infection.[63] If such defenses exist, they could lead
to development of biological prevention strategies. Although a wide variety of
innate mucosal defenses have been described (eg, defensins, mucus, zinc,
amines, etc), none has demonstrated an ability to prevent HIV transmission.
However, "normal" vaginal flora may reduce HIV transmission; women
with flora changes characterized as bacterial vaginosis (ie, increased
anaerobic bacteria, decreased lactobacilli) have higher rates of HIV
acquisition compared with control groups or even with individuals with STDs
(see below).[64,65]
Acquired immune responses to HIV include specific anti-HIV-1
antibodies and cell-mediated immune responses. From studying exposed,
uninfected women, Mazzoli and colleagues[66] reported an association between
increased concentrations of vaginal immunoglobulin A directed against the HIV
envelope and reduced risk of HIV infection. Kaul and colleagues have reported
similar findings with exposed, uninfected commercial sex workers in Nairobi.[67] In addition, both research groups
suggest a role for specific cytotoxic lymphocytes harvested from the peripheral
blood.[68]
These lymphocytes are directed at HIV epitopes that may be conserved across
clades.[68]
In earlier work, Pinto and coworkers reported observing a cytotoxic response in
lymphocytes harvested from health care workers exposed to HIV-1.[69] Not surprisingly, the cytotoxic
T-lymphocyte response is being used as a critical milestone in development of
preventive HIV vaccines (see below).
Acquisition of HIV is reduced in men who have been circumcised.[70,71] In the study by Quinn and colleagues,[22] for example, no circumcised man acquired
HIV. The feasibility of circumcision as an HIV prevention intervention is
discussed later in this review.
Cofactors That Amplify HIV Transmission
A variety of cofactors appear to amplify
HIV transmission by increasing the infectiousness of the index case, increasing
the susceptibility of the exposed host, or both.[1,26] Some of these cofactors are summarized
in Table 1.[1] The most important cofactors are those that cause trauma
or inflammation to the genital mucosa. Classic STDs (genital ulcers and mucosal
inflammatory diseases) occur in the same geographic areas as HIV, and
compelling epidemiological evidence supports the view that such diseases
increase HIV transmission; indeed, the interaction between classic STDs and HIV
is referred to as "epidemiological synergy."[26] For example, Gwanzura and colleagues[72] reported that herpes simplex virus type
2 (HSV-2) appears to increase heterosexual HIV transmission; given the
frequency of HSV-2 infection, this cofactor may ultimately prove to be very
important. Biological studies suggest that STDs may enhance HIV transmission by
increasing the concentration of HIV in genital secretions,[73] the number of cells receptive to HIV,[74] or the number of receptors per cell.[40]
Table 1. Biologic Host-Related Factors Affecting Sexual
Transmission of HIV.*
|
Biologic Factor |
Host-Related Infectivity Factors |
||
|
|
HIV Concentration in Genital Secretions |
Infectiousness (Transmission) |
Susceptibility (Acquisition) |
|
Mutation of chemokine-receptor gene |
? |
? |
|
|
Late stage of HIV infection |
|
|
Not
applicable |
|
Primary HIV infection |
|
|
Not
applicable |
|
Antiretroviral therapy |
|
|
|
|
Local infection (inflammation or ulcer
of reproductive tract or rectal or oral mucosa) |
|
|
|
|
Presence of cervical ectopy |
|
|
|
|
Presence of foreskin |
? |
|
|
|
Method of contraception Barrier Hormonal
contraceptives Spermicidal
agents Intrauterine
devices |
Not
applicable
? ? |
? |
|
|
Menstruation |
? |
|
|
|
Factors that lower cervicovaginal pH |
|
|
|
|
Immune activation |
|
|
|
|
Genital tract trauma |
|
|
|
|
Pregnancy |
|
|
|
*The associations represented were statistically significant in at least one
study. The degrees of positivity (![]() to
to ![]()
![]()
![]() )
and negativity (
)
and negativity (![]() to
to ![]()
![]()
![]() )
of the associations are indicated with arrows, with three arrows indicating a
very strong association. The symbol
)
of the associations are indicated with arrows, with three arrows indicating a
very strong association. The symbol ![]() denotes that there is evidence in
support of both a positive and a negative association. A question mark
indicates an unknown or hypothesized association that is not currently
supported by data.
denotes that there is evidence in
support of both a positive and a negative association. A question mark
indicates an unknown or hypothesized association that is not currently
supported by data.
Royce RA,
Sena A, Cates W Jr, Cohen MS. Current concepts: sexual transmission of HIV. N
Engl J Med. 1997;336:1072-1078. Copyright 1997. Massachusetts Medical Society.
All rights reserved.
A study in Malawi of HIV-infected men with urethritis found that
levels of HIV in their semen were 10 times higher than in subjects without
urethritis (Figure 3).[73] After single-dose antibiotic treatment, HIV excretion
gradually decreased to a level similar to the control group. Likewise, men with
genital ulcers also demonstrate increased excretion of HIV in semen.[75] Phenotypic analysis of HIV in semen
compared with blood suggests that the increased levels of HIV caused by genital
tract inflammation result from local replication, perhaps influenced by local
cytokines.[76-78] It is not yet clear whether the observed increase in HIV
excretion results from increased numbers of infected cells, increased
replication per cell, or both.
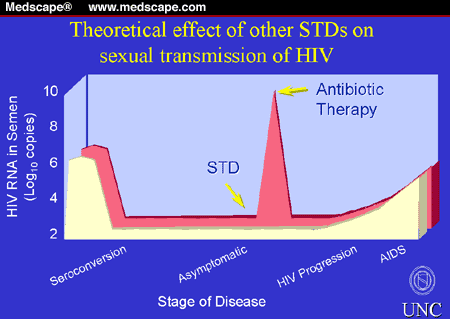
Figure 3. HIV excretion in semen of HIV-infected
men with and without urethritis.
Sexually transmitted diseases also increase excretion of HIV in
female genital secretions,[79,80] which can be reduced by effective
antimicrobial therapy.[80] HIV excretion in women is also influenced by hormones, and
oral contraceptives may have a highly significant effect.[81,82] Medroxyprogesterone acetate (Depo-Provera)
use by commercial sex workers in Kenya resulted in an increased risk of HIV
acquisition in a multivariate analysis. In addition, use of oral contraceptives
was associated with an increased relative risk of HIV acquisition that did not
reach statistical significance in a multivariate analysis.[83] Hormones can also increase
susceptibility. Using macaques, Sodora and coworkers[43] demonstrated a considerable increase in
susceptibility to SIV in animals treated with progesterone, albeit in doses and
a schedule inconsistent with contraception. Conversely, estrogen therapy
reduces the likelihood of vaginal acquisition of SIV.[84]
Two studies have examined the relationship between the presence of
STDs and cells that are receptive to HIV infection. Using cervicovaginal
lavage, Levine and coworkers[74] found an increase in CD4+ lymphocytes in women with a variety
of STDs. Patterson and colleagues[40] noted increased concentration of CCR-5 messenger RNA in
endocervical biopsy specimens harvested from women with STDs. As indicated
above, women with bacterial vaginosis are at greatly increased risk of acquisition
of HIV. Bacterial vaginosis cannot be readily reversed with antibacterial
therapy in women in developing countries.[83]
Intervention Strategies
As indicated above, sexual transmission of
HIV has been described in a mathematical model as Ro = beta x C x delta:, where
beta is efficiency of transmission, C is partner change rate, and delta is
duration of infectiousness.[8] When Ro exceeds 1, new secondary cases of HIV occur, so
successful prevention strategies must reduce Ro to less than 1.
To accomplish this goal, prevention strategies should include
overlapping behavioral and biological approaches. Behavioral interventions aim
to lower the rate of partner change (or to reduce the number of partners with
whom risk behaviors take place), and biological interventions aim to reduce the
efficiency of transmission or to shorten the duration of infectiousness.
Comprehensive HIV prevention strategies are now multisectoral and are aimed not
only at individuals but at families, communities, institutions, and the contextual
barriers to behavior change.[85-90] Approaches have been broadened to
address the social, political, infrastructural, and environmental factors that
influence risk behaviors.[89,90]
Behavior Change Interventions
Perhaps the most difficult area of STD/HIV
prevention lies in the area of behavior change. Behavior change is certainly
difficult to inspire and extremely hard to measure. Furthermore, the
theoretical basis for behavior change has been difficult to characterize.
Challenges in behavior change programs include (1) the most effective way to
measure intermediate stages of change to assess a program's effectiveness and
(2) how to sustain behavior change. A comprehensive behavior change
intervention strategy must be designed to address specific target groups in
which HIV is being transmitted,[90] to take account of the stage and the progress of the
epidemic, and to combine an array of media and interpersonal methods. In
addition, behavior changes at the societal level are essential.[89]
Given these limitations, the kinds of behavior changes desired are
obvious. Such changes are intended to reduce acquisition HIV infection by
promoting the following:
- sexual
abstinence
- delayed
sexual debut
- sexual
monogamy
- correct
and consistent condom use
- avoidance
of injection drug use or safe drug-injecting habits
- health-seeking
behavior for rapid treatment of sexually transmitted infections
Methods used to inspire behavior change
range from intensive education aimed at individuals to mass advertising as offered
on commercial television. Behavior change interventions generally draw on
contemporary marketing principles such as the following:
- audience
segmentation based on variables such as age, sex, attitudes, and values
- audience
research
- concept
development and pretesting
- multiple
message development to target various segments of the audience
- design
of messages that offer benefits meaningful to the audience, ensuring
accessibility to needed services or products.[91]
Behavior change has been documented in
high-risk individuals both in the United States and in developing countries.[92,93] It is clear that as early as the
mid-1980s, before the initiation of large-scale public education campaigns, gay
men enrolled in cohort studies modified their sexual behavior in response to
growing awareness of the existence of AIDS and education campaigns mounted by
gay community-based groups. However, only more recently have different types of
prevention intervention been rigorously evaluated in properly controlled settings,
which have begun to shed light on the type and frequency of interventions
required.
Examples of controlled studies of prevention interventions include
the following:
Project Respect. follow-up study is now under way.
San Antonio study. Investigators in San Antonio, Texas,
enrolled 617 Mexican American and black women in a randomized study comparing 3
small-group behavioral-cognitive interventions vs standard counseling about
STDs. Rates of chlamydial and gonorrheal infections were significantly lower in
the intervention group at 6 and 12 months.[93]
Project Light. This randomized controlled trial
compared a 1-hour education session with a 7-session program focused on
attitudes about safer sex, skills building, and risk reduction strategies and
enrolled 3706 people at 37 STD or primary care clinics across the United
States. During 1-year follow-up, participants who received the 7-session
intervention reported less unprotected sex, increased condom use, and more
consistent condom use.[94]
The HEROES Project. An alternative strategy for producing
individual behavior change is to attempt to influence group norms. Kelly and
colleagues[95] evaluated this type of HIV prevention intervention in 3
different cities in sequence. Popular trendsetters who frequented gay bars and
clubs were trained in peer education techniques and contracted to communicate
risk reduction recommendations and endorsements to their friends and
acquaintances. In each of the cities, the incidence of unprotected anal
intercourse after intervention decreased by 15% to 29% compared with baseline
levels.
Successes in developing countries such as Thailand and Uganda
deserve special attention and are discussed in the context of condom use below.
Prevention Initiatives for HIV-Infected Individuals
Ever since the recognition that AIDS is
caused by HIV infection, HIV prevention initiatives developed by public health
agencies in the United States have focused almost entirely on encouraging
"harm reduction" behavior in HIV-negative risk groups or the entire
US population. While this is an important objective, prevention services for
HIV-infected individuals are also essential.[96] At the beginning of the epidemic,
diagnosis of HIV infection resulted in intense stigma with virtually no
personal medical benefit. However, with the availability of effective,
life-saving treatment it makes perfect sense to try to detect virtually all
HIV-infected people, so that provision of treatment could be combined with
strategies to prevent further transmission. Furthermore, as discussed in this
review, most experts believe that treatment with highly active antiretroviral
regimens reduces an individual's infectivity, decreasing the probability of HIV
transmission.
Recognizing the importance of this approach, in February 2001 the
US Centers for Disease Control and Prevention (CDC) launched a new prevention
strategy called Serostatus Approach to Fighting the HIV Epidemic (SAFE). This
project has 5 goals:
- To
create an environment in which people know their HIV status. A variety of
studies suggest that people who know their status are more likely to
engage in safer sex behavior. To achieve this goal, the CDC is launching a
campaign entitled "Know Now" which will use radio ads in HIV
high-transmission areas to inspire voluntary testing.
- People
with newly diagnosed HIV infection will need to be provided easy access to
healthcare. Some studies have demonstrated a long lag time between
recognition of infection and appropriate healthcare.
- HIV-infected
subjects need to be provided with appropriate therapy at reasonable cost.
- Adherence
to therapy must be emphasized.
- Safer
sex behavior in this population must be achieved and sustained.
The CDC estimates that perhaps
200,000-300,000 HIV-infected people are unaware of their status, and an equal
number of HIV-infected people remain untreated. In addition, there are 40,000
new (incident) cases of HIV each year in the United States. The CDC hopes to
increase the percentage of infected people who know their status to 90%, and to
reduce incident cases by 50% by 2005.
The CDC plans to garner $300 million for this program from its own
resources and through collaborative partnerships with other governmental
agencies. Sadly, these resources will likely fall far short of what is required
for success. In addition, the program may not sufficiently emphasize the
critical importance of healthcare workers in the private sector. More than 90%
of prescriptions for HIV drugs are written by only 2000 doctors. These doctors
have no incentive to participate in HIV prevention programs, nor have they
received any training for this purpose. Indeed, there are almost no data to
inform the best approach for prevention in HIV-infected people. Conversely,
there are compelling data to show that HIV-infected people often continue to
engage in unprotected intercourse, putting their partners at grave risk.
The latter issues were addressed extensively in a CDC-supported
report[97]
by the Institute of Medicine designed as a comprehensive blueprint for HIV
prevention. An entire chapter of this report is devoted to prevention of
transmission from HIV-infected people, with considerable emphasis on strategies
to draw in, re-educate, and compensate treatment providers from the private
sector. The latter approach may ultimately be most critically important to the
success of SAFE.
Focusing on HIV-infected individuals can have a dramatic effect on
the epidemic but should be pursued within an ethical framework that respects
both the rights and responsibilities of infected individuals. Cuba appears to
have avoided an HIV epidemic, at least temporarily, through mass screening and
quarantine and re-education of HIV-infected individuals, an approach used by
other totalitarian countries to control STDs.[98]
Obstacles to Individual Behavior Change
Although considerable progress has been
made in this arena, population level factors may ultimately prove limiting both
in the United States and abroad. Studying sexual behavior in black women,
Adimora and coworkers[99] proposed that risky sexual behavior among this population
is largely secondary to the unavailability of appropriate male sexual partners.
The disproportionate number of black men incarcerated and the low birth rate
and high death rate in this community essentially force women to share men.
Change in individual behavior becomes a less effective intervention in this
setting.
In developing countries already ravaged by HIV, another problem
surfaces. HIV prevention strategies are incompatible with conceiving children,
and the high prevalence of HIV means that transmission commonly occurs as a
direct result of attempts toward reproduction. In this context, the
availability of therapy to reduce vertical transmission of HIV is also a
critical health consideration but one destined to increase the number of
"AIDS orphans" already so visible throughout sub-Saharan Africa.
Improving the Availability and Use of Condoms
The effectiveness of condoms in preventing
many STDs has been well established.[100] The ability of condoms to prevent HIV
transmission has also been extensively evaluated. Ten cohort studies conducted
among high-risk populations in 7 countries, in which both the level of condom
use (HIV exposure) and HIV incidence (outcome) were prospectively measured,
have shown that consistent use of condoms by men reduces HIV acquisition by
between 50% and 100%.[101] Two studies involving HIV-discordant
couples were compelling. In a study of serodiscordant couples in Europe, none
of the 123 HIV-negative partners who prospectively reported consistent condom
use became infected.[102] In Haiti, only 1 of 42 seronegative
partners who consistently used condoms with their seropositive partners became
infected.[103] Among HIV-discordant couples who either used condoms
inconsistently or did not use them at all, between 7% and 14% became infected.
Targeting condom use to populations where HIV is spreading rapidly
can have a marked impact. The 100 Percent Condom Program in Thailand is a
remarkable success story. In 1991, the Thai government implemented a national
strategy to encourage condom use in commercial sex facilities. The proportion
of commercial sex acts in Thailand where condoms were used increased from 25%
in 1989 to 94% in 1995. During the same interval, the incidence of curable STDs
reported to the government clinics decreased dramatically, as did HIV
prevalence among Thai military recruits.[104] In the United States, Nevada brothels
that have instituted similar policies have not had a single reported case of
HIV transmission during the epidemic in this country.[105]
The latex condom for men is available in many countries through
private pharmacies, maternal and child health and family planning clinics, STD
services, community-based distribution systems linked with peer education
programs, and condom social marketing initiatives. The latter have been
extremely successful in vastly improving access to condoms by making them
available to consumers at an affordable, usually subsidized price and in
convenient, nontraditional outlets, such as bars, taxis, and hotels. For
example, condom sales in Ethiopia increased from 300,000 in 1990 to 19.8
million in 1995, with more than 90% of the condoms sold in nontraditional
outlets. Moreover, the aggressive promotion of condoms in condom social
marketing programs makes them more acceptable. Social marketing approaches are
also being used to increase use of STD treatment and prevention services.[106]
Although the latex condom for men is a key element in HIV
prevention programs, it can only be used at the discretion of the male partner.
"Female condoms" for women are now available in some countries. Their
efficacy against HIV transmission has not been proven. The female condom for
women is currently expensive (several times the cost of a latex condom for men)
and can only be used once. The high cost and issues of acceptability have
limited its use. However, in a group of 99 high-risk couples in Zambia who were
counseled and given supplies of female condoms, spermicides, and male condoms,
during 1-year follow-up the female condom continued to be used by most couples
and was used in about one quarter of the coital acts.[107]
Increasing Health-Seeking Behavior
In addition to reduced partner change and
condom use, a third component of desirable behavior change is improved
health-seeking behavior. This is considered likely to affect the rate of sexual
HIV transmission, because people exposed to HIV may be at increased risk of
infection if they have an untreated STD or other cause of inflammation[26] and because people with HIV may transmit
the virus more efficiently if they have an STD. Cost-effectiveness analysis is
necessary to determine which STDs deserve the most attention, given limited
resources.[108] In many cases, currently available research results are
insufficient to enable us to reach firm conclusions.
Biological HIV Prevention Strategies: Reducing
Infectiousness, Susceptibility, and the Efficiency of Transmission
Biological interventions to prevent sexual
transmission of HIV include interventions to reduce susceptibility (eg,
vaccines, topical microbicides) and infectiousness and/or susceptibility (eg,
elimination of transmission cofactors, use of antiretroviral therapy).
Vaccines
Vaccines are clearly the most
cost-effective way to prevent transmission of infectious diseases. Development
of vaccines is particularly important for STDs, since change in sexual behavior
is so difficult to accomplish and document.[109] Unfortunately, vaccine development is
time-consuming and expensive. An ideal vaccine for HIV would work by increasing
immunity of the susceptible host to prevent mucosal or systemic infection
(primary prevention). Even a vaccine that did not completely protect against
infection but that did provide enough immunity to reduce the initial viral
burden (secondary prevention) could make a significant contribution to limiting
the epidemic by making transmission to the next sexual partner less likely.
Conversely, there is concern that a vaccine that is less than 100% effective
could increase overall rates of HIV transmission if it resulted in an increase
in risk-taking behavior.[110] Such a vaccine might also increase the
incidence of classic STDs.
Work on developing a vaccine against HIV began as soon as the
pathogen was identified, but it has proved a daunting task because of the
variability of the virus and our limited understanding of the immune responses
required to prevent infection. Also, vaccines have been generated using
antigens derived from viral strains in blood, which are not always the same as
antigens recovered in semen.[111] Vaccine efforts to date have focused
primarily on the clade B virus represented in the United States and Western
Europe, whereas other viral clades (especially clades A, E, and C) predominate
in the epicenters. It will take a considerable time to complete studies of
clade B vaccines in the United States because of the low prevalence and limited
transmission of HIV in that country. Accordingly, trials of vaccines against
clade B and other clades will also be conducted in Uganda, Thailand, and other
developing countries.
Vaccines generally include critical viral immunogens (ie, pieces
of the HIV envelope, gag, or other proteins) delivered directly with an
adjuvant or by means of genes inserted in a live viral vector to improve the
immune response.[112] Several novel approaches, such as DNA
vaccines and alternative vectors and adjuvants, are also being evaluated. The
appropriate immunological milestone required to justify full-scale
effectiveness trials is not known, since a protective immune response has not
been documented -- the work of Clerici's group and others notwithstanding.[66] Nevertheless, cytotoxic lymphocyte
responses and serum neutralizing antibody responses have been carefully studied
in vaccine recipients and have been selected de facto as
surrogates of immunity. There is little evidence to support a role for
immunoglobulin G antibody-mediated protection from HIV. Also, the cytotoxic
lymphocyte response being measured is extremely difficult to quantitate and has
limited duration in vaccine recipients.
Prevention of sexual HIV acquisition must depend on immune
defenses present at mucosal surfaces. However, clinical HIV vaccine development
to date has focused almost entirely on systemic immune responses after
inoculation. In large part, this disconnection between the research being
undertaken and the data we actually need may be due to the extreme complexity
of studying mucosal immunity. Collection of specimens is difficult, and the
samples acquired are always limited. In addition, few vaccinologists or
immunologists have expressed interest in mucosal immunity. Despite the enormous
interest and investment in an HIV vaccine, the study of mucosal immunity
remains in its infancy.
Topical Microbicides
As noted in the earlier discussion of
condoms for women, there is a critical need for products that could be used by
women to protect themselves from STDs and HIV. Such products must be active
against a range of sexually transmitted pathogens, have very few toxic effects,
allow reproductive function, and be acceptable for sexual behavior.
Idealistically, such products would be biodiffusable and bioadhesive and have
long duration of effect. In addition, such products should leave vaginal flora
unchanged. They must not be absorbed systemically, and they must be able to be
maintained at room temperature so that no special storage is required.
Currently, the only topical microbicide in widespread use is
nonoxynol-9 (N-9), and its use is now contraindicated, as discussed below.
However, a variety of products in different categories are in development.
These products include the following:
- other
detergents in various formulations
- polymers
that are designed to block HIV attachment
- compounds
that would be expected to change the pH of the vaginal fluids
- antiretroviral
agents
- suppositories
designed to increase the concentration of hydrogen peroxide-producing lactobacilli
in the vagina, in the belief that such bacteria contribute to innate
resistance to HIV and other STD pathogens
N-9 is the most well-studied product for
the prevention of acquisition of HIV and other STDs. This compound was
developed as a spermicide without consideration of its effects on vaginal flora
or disease acquisition. Randomized controlled trials have compared 3 different
N-9 products: a gel,[113,114] a sponge,[115] and a film.[116] These studies have shown that N-9
slightly reduces the risk of gonorrhea or chlamydial infections. In 2 studies
that looked at HIV acquisition, one found a trend toward increased HIV
transmission with increased frequency of genital ulcers and vulvitis,[115] whereas the other found no effect on the
rate of acquisition of HIV but again, an increased rate of genital lesions.[116]
The controversy over the use of N-9 controversy was effectively
ended at the XIII International AIDS Conference in Durban, South Africa, in
July 2000. Lut van Damme[118] reported that in a multicenter trial
women using an N-9 gel had a higher rate of HIV acquisition than women using a
placebo gel. Exposed women were also more likely to have vaginal inflammation.
No protection from STDs was observed. Work from Hoffman and colleagues[119] further confirmed that inflammation is
observed with frequent use of N-9. The van Damme study[118] can be criticized for loss to follow-up
and the lack of a true, third-arm control group using no topical application
(to address the possibility that the placebo gel had an effect). However, the
results suggest that inflammation caused by detergents applied frequently may
offset any antiviral benefit observed in vitro.
There are other special problems encountered in trials of topical
microbicides. First, the use of a placebo is a challenge if the preservative(s)
present in the placebo could affect the vaginal flora. Second, condom use,
which must be recommended to all subjects, complicates interpretation. Third,
product use is difficult to record. Finally, men are exposed to these products
without informed consent. These limitations notwithstanding, the need for a
topical microbicide is evident, and ongoing or planned trials are likely to
generate important results.
Treatment of Cofactors That Facilitate Transmission
The concentration of HIV in the genital
tract is increased by vitamin A deficiency,[117,120] high-dose oral contraceptives,[117] and classic STDs.[73,79,121] In addition, STDs probably increase
susceptibility to HIV by reducing mucosal resistance to HIV and recruiting
cells that are receptive to infection with HIV to the genital mucosa.[40,74] Recent studies suggest that the changes
in the vaginal flora that characterize bacterial vaginosis also increase
women's susceptibility to acquiring HIV.[64,65]
Several studies have demonstrated that treatment of STDs reduces
excretion of HIV.[117] Syndromic algorithmic treatment of STDs
in Tanzania reduced incident cases of HIV by 42%,[122] an extremely cost-effective approach to
HIV prevention.[123] In Wellcome, South Africa, monthly use
of azithromycin by sex workers serving a mining community led to lower levels
of gonorrhea and Chlamydia in both the sex workers themselves
and their male clients.[124] In rural Uganda, mass therapy
administered every 9 months to community-based sociosexual networks decreased
the incidence of gonorrhea, syphilis, and trichomoniasis[83]; however, HIV transmission was not
reduced. The difference between the studies in Uganda and Tanzania may reflect
the enrollment of different populations with different STDs.[125] In addition, the study in Tanzania
focused on treatment of symptomatic STDs, which may be of greatest importance
in HIV transmission. These concerns notwithstanding, it is hard to argue
against detection of treatment of STDs, which cannot help but benefit
reproductive health.
Circumcision
Some classical STD pathogens such as
gonorrhea and chlamydia infect the anterior urethra, whereas those that cause
ulcers (eg, syphilis) infect the shaft or the mucosa of the glans penis. Two
lines of evidence suggest that HIV might be transmitted to men in the same way
as pathogens that cause ulcers: (1) men with genital ulcers appear to have an
increased susceptibility to HIV infection,[26] and (2) dendritic cells receptive to HIV
can be found in the mucosal surface of the glans.[126] When men are circumcised, the mucosal
tissue of the glans keratinizes and evolves into stratified squamous
epithelium, which would be expected to be more resistant to STD pathogens. The
foreskin itself may represent an important route by which HIV can gain access
to the body. Bailey and coworkers[127] have shown that the foreskin contains a
variety of the cellular elements potentially important for HIV acquisition and
can be successfully infected with HIV in vitro. Langerhans cells represent 4.7%
of foreskin cells and express both the CD4 and the CCR5 receptors required for
HIV acquisition.
An entire session of the XIII International AIDS conference in
Durban, South Africa, in July 2000 was devoted to discussion of circumcision to
prevent acquisition of HIV. Evidence of the benefit of circumcision was
provided by Buve and coworkers.[128] They reported on a community-based,
cross-sectional study comparing African communities with high and low HIV
prevalence. Two thousand people were studied in each of 4 towns. Subjects were
interviewed, examined, and tested for STD pathogens and HIV. The investigators
found that in Yaounde, Cameroon, and Cotonou, Benin, the prevalence of HIV was
3.8% and 4.4%, respectively. Ninety-nine percent of the men studied were
circumcised. Conversely, in Kisumu, Kenya, and Ndola, Zambia, where the HIV
prevalence was 21.9% and 25.9%, respectively, only 26.8% (Kisumu) and 7.6%
(Ndola) of men were circumcised. Statistical analysis, including adjustment for
sexual behavior, marital status, ethnic group, herpes simplex virus-2
antibodies, and syphilis, demonstrated that circumcision appeared to provide
significant protection from HIV acquisition. The authors concluded that at
least some of the regional variation in HIV prevalence in Africa could be
ascribed to circumcision, and that this procedure might be introduced as an HIV
prevention measure.
Using a very different study design, Gray and coworkers[129] also reported on the protective effects
of circumcision. These investigators studied HIV acquisition in a cohort of
5507 men in rural Uganda. Of the men in the study, 16.5% were circumcised.
Ninety-nine percent of the circumcised men were Muslims; 3.7% of non-Muslims
were circumcised. Circumcision was associated with a significant decrease in
acquisition of HIV in the cohort. Because Muslim and non-Muslim men may engage
in very different sexual behaviors, the study results must be interpreted with
caution. The time of circumcision was important because protection was observed
only in men who were circumcised before puberty. A total of 187 HIV-negative
men were in sexual partnerships with HIV-infected women. In the discordant
couples, none of 50 circumcised men acquired HIV from his female partner. HIV
was acquired in the uncircumcised men at a rate of 16.7 per 100 patient-years.
Controlled trials to evaluate the benefit of circumcision may be
necessary. In preparation for such trials, Taljaard and coworkers[130] evaluated social factors affecting the
likelihood of circumcision in a community in South Africa. The data emphasize
the wide variation in circumcision and the reasons for circumcision in
different tribal groups. Some groups use circumcision as part of a ritual of
manhood, perhaps too late to offer protection from HIV. The potential health
benefits of circumcision were also widely appreciated by respondents in this
study.
In preparation for an intervention trial, acceptance of
circumcision was studied in the Nyanza Province in Kenya, where circumcision is
not traditionally practiced. Bailey and colleagues[131] interviewed 106 men, 110 women, and 45
clinicians. The results strongly suggest that this community would accept
circumcision. Most respondents thought the procedure should be performed on
boys at 8.6 years (range, 1-22 years). This group now intends to conduct a
randomized, controlled trial of circumcision in Kenya. The study will compare
HIV acquisition rates in 1000 control subjects who will remain uncircumcised
with those of 1000 subjects who will be circumcised as part of the study. The
investigators estimate that a study of this size will demonstrate the benefits
of the approach within 2 years. The study will enroll men over 18 years of age,
which represents a potential limitation because some investigators have
indicated that circumcision has its greatest impact on rates of HIV acquisition
when performed before puberty. The study will also determine the costs,
limitations, and complications of circumcision in a resource-poor setting.
These latter factors have been a source of concern to many
authors,[70,71,132] especially with regard to circumcision of adults. It will
be important for these costs to be compared with the benefits expected.
Developing an infrastructure and public and governmental support for routine
circumcision in infants, children, or adults would be no small undertaking, and
the benefit (ie, protection from HIV) would not be realized for years or
decades. This being said, many countries suffering from an HIV epidemic (or
threatened epidemic) that is likely to continue for decades would do well to
consider this intervention now.
Antiretroviral Therapy and HIV Transmission
As discussed earlier, it is increasingly
clear that the concentration of HIV in genital secretions helps to determine
the efficiency of transmission. There are 2 ways that antiretroviral drugs may
be used to prevent transmission:
- First,
use of effective antiretroviral therapy by index cases typically results
in a reduction in HIV in blood, semen, and, possibly, vaginal secretions
and thus may be expected to reduce infectivity. In a retrospective study,
Musicco and coworkers[133] reported that when index cases were
receiving the antiretroviral drug zidovudine transmission to exposed
partners was reduced. However, the ability of antiretroviral drugs to
reduce sexual transmission of HIV has not been demonstrated prospectively.
- Second,
antiretroviral agents can be given to uninfected people as pre-exposure or
postexposure prophylaxis. However, there are no data on the efficacy of
these strategies in preventing sexual HIV transmission in humans.
Availability of antiretroviral therapy for
prevention could lead to increased risk-taking behavior in both HIV-positive
and HIV-negative individuals. There have been a number of anecdotal reports
suggestive of such increased risk taking among gay and bisexual men. Several
studies examining this possibility are in progress.
Antiretroviral Drugs to Reduce Infectiousness
The benefits of antiretroviral drugs for
HIV-infected patients have been extremely well documented. Potent combinations,
including nucleoside analogue reverse transcriptase inhibitors (NRTIs),
nonnucleoside RT inhibitors (NNRTIs) and protease inhibitors, can be expected
to drastically reduce the viral burden in semen and blood. However, as
described earlier, differences between HIV isolates from genital secretions
compared with blood strongly suggest that virus in the genital tract resides in
a unique compartment.
The male ejaculate is composed of secretions from testes (10%),
the seminal vesicles (50%-70%), and the prostate (20%-30%). Secretions from the
urethra, epididymis, and ampulla contribute 10% to the overall ejaculate
volume. The exact mechanisms for distribution of drugs into these secretions
are not known. It seems most likely that the boundary between the blood and
semen is a lipid barrier and that most drugs will enter into the secretions by
passive diffusion. Physical chemical properties likely to regulate the rate at
which compounds passively diffuse from blood into semen include the
dissociation constant, partition coefficient, and plasma protein binding. Table
2 summarizes these parameters for existing antiretroviral drugs and the
predicted concentrations of antiretroviral drugs in different seminal
secretions based on available data. In addition, active transport of compounds
into genital secretions is possible, as mediated by adenosine
5'-triphosphate-dependent p-glycoprotein transporters. The effects of
p-glycoprotein transporters on the concentrations of HIV in genital secretions
is not known.
Table 2. Physicochemical and Pharmacokinetic Parameters of
Representative Antiretroviral Agents Potentially Influencing Seminal-Compartment
Distribution
|
Drug |
pKa |
Lipid solubility (partition coefficient) |
Time to C max a (h) |
Elimination half-life in serum (h) |
Protein binding (%) |
|
Nucleoside reverse transcriptase Zidovudine Lamivudine Didanosine Zalcitabine
Stavudine Abacavir |
4.3 9.1 4.4 10.0 0.4, 5.1 |
Hydrophilic (NA b ) Slightly lipophilic (NA) Hydrophilic (0.04) Hydrophilic (NA) Lipophilic (NA) |
1-1.5 0.5-4.6 0.5-2 3.8 0.7-1.7 |
3-5 0.6-2.9 1-3 1-1.5 0.9-1.7 |
10-50 < 5 < 4 Negligible 50 |
|
Nonnucleoside reverse transcriptase Nevirapine Delavirdine
Efavirenz |
4.3-4.6 10.2 |
Slightly lipophilic (2.98) Lipophilic (NA) |
1 2-5 |
4.4-11 40-52 c |
98 99.5 |
|
Protease inhibitors Ritonavir Saquinavir Indinavir Nelfinavir Amprenavir |
2.8 1.1, 7.1 6.2 6.0, 11.1 1.9 |
Lipophilic (4) Lipophilic (NA) Hydrophilic (NA) Lipophilic (NA) |
2-4 NA 0.5-1.1 2-4 1.2 |
3-5 6 1-3 3.5-5 7-10 |
98 > 98 60 99 95 |
a
C max, maximum concentration of drug in serum.
b NA, not available.
c At steady state.
Kashuba AD,
Dyer JR, Kramer LM, Raasch RH, Eron JJ, Cohen MS. Antiretroviral-drug
concentrations in semen: implications for sexual transmission of human
immunodeficiency virus type 1. Antimicrob Agents Chemother. 1999;43:1817-1826.
Copyright 1999. American Society for Microbiology. All rights reserved.
Only a limited number of studies have been undertaken to determine
the actual concentrations of antiretroviral drugs in semen (reviewed in the
paper by Kashuba and associates[44]). Henry and colleagues[134] studied 6 men who received 800 to 1200
mg of zidovudine per day. The ratio of semen concentrations of zidovudine to
serum concentrations ranged from 1.3 to 20.4, demonstrating that zidovudine is
concentrated in male genital secretions. These results suggest that there may
be active transport or trapping of zidovudine in the male reproductive tract
and that the theoretical calculations in Table 2 may be
inaccurate. We studied 71 semen and plasma samples collected from 9 men during
the first 200 days of antiretroviral therapy. The blood-plasma ratio of
zidovudine was 5.9 (25th to 74th percentile, 0.95-15.5). For lamivudine, the
blood-semen ratio was 9.1 (2.3-16.1).[135]
More recently, ritonavir and saquinavir have been studied. These
agents appear to achieve very low concentrations in seminal plasma.[136] Amprenavir and indinavir appear to
penetrate into the male genital tract, and indinavir concentrations were
enhanced by ritonavir.[137,138] The poor penetration of some protease
inhibitors into seminal plasma might be ascribed to high levels of protein
binding or other factors that remain unknown.
Kim and colleagues[139] conducted a detailed study of the
penetration of efavirenz into semen. Blood and semen samples from 14 men were
collected 6 times during a 24-hour period postdose. Efavirenz achieved 2-fold
higher concentrations in seminal plasma than in blood. The blood plasma area
under the curve (AUC) was predictive of the seminal plasma AUC, and AUC
measurements were more reliable than random ratios.
For the NRTIs, the critical parameter is the intracellular level
of the active metabolite, after appropriate phosphorylation. Although the
positive effects of antiretrovirals in genital secretions (see below) would
strongly suggest that phosphorylated products are available, phosphorylation in
seminal cells has not been directly measured. Indeed, by examining this
question it might be possible to determine whether antiretroviral drugs are
inhibiting HIV in the semen itself or before it ever gets into the semen.
To date, 16 studies on the effects of antiretroviral drugs on HIV
in semen have been published (Table 3). When the most potent combinations of
drugs were used, HIV-1 could only rarely be detected in seminal plasma.
However, 2 studies demonstrated that HIV-1 DNA can be recovered from seminal
cells despite suppression of HIV-1 RNA in blood and seminal plasma[45,46]; in one study, infectious virus was
recovered from the seminal cells of some men who had prolonged HIV-1
suppression with therapy.[46]
Table 3. Summary of Published Studies Evaluating the
Effects of Antiviral Therapy on HIV-1 Shedding in Semen a
|
Reference [yr] |
n |
Study type |
Drug therapy and no. of subjects |
|
Anderson et al. [1992] |
95 |
Cross-sectional |
None, 64; AZT, 31 |
|
|
14 |
Longitudinal (5-8 mo) |
None, 9; AZT, 5 |
|
Delwart et al. [1998] |
5 |
Cross-sectional |
None, 2; AZT, 2; AZT + ddI, 1 |
|
Eron et al. [1998] |
11
(5 ARV na´ve, 6 NA experienced) |
Longitudinal (8-58 wk) |
Na´ve patients, single or dual NA or NA |
|
Gilliam et al. [1997] |
11
on newly initiated therapy, 11 on stable therapy |
Longitudinal (up to 90 wk in the new TX
group, up to 26 wk in the stable TX group) |
New TX; AZT or ddI ▒ DLV; stable TX; no
TX, 6; mono-TX, 1; combination TX, 4 |
|
Gupta et al. [1997] |
6 |
Longitudinal (0-28 wk) |
IND ▒ efavirenz |
|
Hamed et al. |
36 |
Cross-sectional |
ART na´ve, 9; AZT, 19; ddl, 4; AZT +
ddl, 4 |
|
|
6 |
Longitudinal (8 wk after TX initiation) |
AZT or ddl, AZT + ddl, or AZT + ddC |
|
Krieger et al. [1991] |
34 |
Cross-sectional |
None, 30; AZT, 22 |
|
Krieger et al. [1995] |
56 |
Mixed (single samples from 22 subjects;
multiple samples from 34 subjects) |
None, 8; AZT or ddI, 30; AZT + ddC 2,
unknown, 16 |
|
Kroodsma et al. [1994] |
16 |
Cross-sectional |
AZT, 7; ddI, 6; AZT + ddI, 3 |
|
Musicco et al. [1994] |
436
couples (HIV-1-infected men and their monogamous seronegative female sexual
partners) |
Cohort (740 person-year follow-up) |
AZT, 64 |
|
Vernazza et al. [1997] |
101 |
Cross-sectional |
ARV-na´ve, 53; 1 or 2 NAs, 29; no
therapy, 19 discontinued |
|
Vernazza et al. [1997] |
4
(19 TX na´ve, 25 experienced) |
Longitudinal (0-10 wk) |
AZT, 1; IND, 1; AZT + 3TC, 5; 2 NAs +
PI, 16; 1 or 2 NAs ▒ SQV or DLV, 21 |
|
Vernazza et al. [1999] |
114
cases, 55 historical controls |
Case control |
Cases: 2 NAs PI, 97; |
|
Zhang et al. [1998] |
7 |
Cross-sectional |
2 NAs + PI for 5-41 mo prior to study |
Table 3
(continued)
|
Reference [yr] |
STD screening |
HIV-1 detection |
Results |
|
Anderson et al. [1992] |
Yes |
Microculture |
Median AZT concn 0.004 (0-0.019) ng/ml;
AZT associated with a decrease in detection of seminal HIV-1 (adjusted OR,
0.04; 95% CI, 0-0.63) |
|
|
Yes |
Microculture |
HIV-1 detected in 43% of men without AZT
TX and in 0% of men with AZT TX |
|
Delwart et al. [1998] |
Unknown |
HIV-1 RNA sequence analyses by nested
PCR |
Distinct variant populations in BP and
SP in 3/5 subjects; no semen-specific signature amino acid sequence detected |
|
Eron et al. [1998] |
Yes |
HIV-1 RNA by NASBA, HIV-1 RNA sequence
analysis by Affymetrix |
Median BP and SP HIV-1 RNA levels before
TX, 5.1 and 5.7 log10 copies/ml; median maximal change in BP and SP HIV-1 RNA
levels with TX, -0.95 and -1.41 log10 copies/ml; baseline SP RT resistance
mutations in 3/6 NA-experienced subjects (vs 1/5 for TX-na´ve subjects); 8/11
subjects developed resistance mutations during TX (to NAs and PIs in BP; to
NAs in SP) |
|
Gilliam et al. [1997] |
Yes |
Microculture of seminal cells, HIV-1 RNA
by NASBA |
Before TX, 50% seminal-cell culture
positive; after TX, 0.1% seminal-cell culture positive; median SP HIV-1 RNA
reduction, 1.01 log10 (78% BLQ); in 55%, decrease in SP HIV RNA was > BP
HIV RNA decrease; in stable TX group, median SP HIV-1 RNA, 1.45 log10 lower than that in patients on TX (P
= 0.05), with less within-subject variability |
|
Gupta et al. [1997] |
Unknown |
HIV-1 RNA by NASBA |
SP RNA decreased 5- to 10-fold after 2
wk (n = 3), SP RNA decreased 4- to 150-fold after 4 wk (n
= 6), HIV-1 RNA remained undetectable in BP and SP for duration of study |
|
Hamed et al. |
Yes |
RT-PCR |
HIV-1 DNA detected in semen in 67% of
untreated patients and 78% of treated patients, HIV-1 RNA detected in semen
in 44% of untreated patients and 48% of treated patients |
|
|
Yes |
RT-PCR |
Before TX, HIV-1 DNA detected in blood
in 6/6 patients and in semen in 4/6 and remained stable over 8 wk of TX;
before TX, HIV-1 RNA detected in BP 4/6 in patients and in SP of 2/6 patients
and decreased to BLQ during 8 wk of TX |
|
Krieger et al. [1991] |
Unknown |
Mixed-lymphocyte culture method, with
HIV p24 antigen detection |
16/55 semen samples HIV positive (10 in
cellular fraction only, 3 in plasma fraction only, 3 in both fractions); no
difference in HIV isolation between TX and no TX (41 vs 23%) or between symptomatic
and asymptomatic (28 vs 32%) |
|
Krieger et al. [1995] |
Yes |
Mixed-lymphocyte culture method, with
HIV p24 anigen detection by ELISA |
36/215 semen samples HIV positive (24 in
cellular fraction only, 5 in plasma fraction only, 7 in both fractions); CD8 + in BP predictive of HIV-1 in semen
(OR, 5.0; 95% CI, 1.0-23.9); TX not associated with HIV-1 in semen (OR, 1.7;
95% CI, 0.6-5.1) |
|
Kroodsma et al. [1994] |
Unknown |
RT-PCR |
Recovery of HIV-1 RNA, 68% in BP and 44%
in SP; 25% of paired semen and blood samples had discordant genotypes (codon
215 or 74) |
|
Musicco et al. [1994] |
Unknown |
Seroconversion measured by ELISA with
Western blot confirmation |
Incidence rates of seroconversion with
and without AZT TX, 4.4 and 3.8, respectively, per 100 person-years; relative
risk of sexual transmission of HIV with AZT TX, 0.5 (95% CI, 0.1-0.9) |
|
Vernazza et al. [1997] |
Yes |
NASBA |
Seminal HIV lower in treated vs
untreated patients (P = 0.03); treatment was an independent
inverse predictor of HIV RNA detection (OR, 0.38; P = 0.054) |
|
Vernazza et al. [1997] |
Yes |
RT-PCR (blood), NASBA (semen), and
microculture |
At baseline, 68% had HIV-1 RNA in SP and
37% had HIV-1 in coculture; at follow-up, 27% had HIV-1 RNA in SP and 12% had
HIV-1 in coculture; the 27% with undetectable BP RNA also had undetectable SP
RNA and culture |
|
Vernazza et al. [1999] |
Yes |
HIV-1 RNA by Nuclisens (114 cases, 55
controls) HIV-1 DNA by nested PCR (67 cases, 55 controls) |
67% detection frequency of HIV RNA in
controls vs 2% in cases (OR, 0.01; 95% CI, 0.0-0.3); 38% detection frequency
of HIV DNA in controls vs 16% in cases (OR, 0.32; 95% CI, 0.12-0.80) |
|
Zhang et al. [1998] |
Unknown |
HIV-1 RNA by RT-PCR, p24 antigen ELISA
of mixed-lymphocyte coculture, DNA sequence analysis |
HIV-1 RNA in BP and SP BLQ; HIV-1 DNA in
PBMC of 7/7 and in seminal cells of 4/7; replication-competent HIV-1 in PBMC
of 3/7 and in seminal cells of 2/3 (1 macrophage tropic and 1 dual tropism) |
a Only Anderson et al. 2 in the cross-sectional
study measured antiretroviral-drug concentrations, in 19 of 31 patients. BP,
blood plasma; SP, seminal plasma; RT, reverse transcriptase; TX, antiretroviral
therapy; NA, nucleoside analogue; PI, protease inhibitor; AZT, zidovudine; ddI;
didanosine; ddC, zalcitabine; IND, indinavir; SQV, saquinavir; DLV,
delavirdine; HU, hydroxyurea; BLQ, below limit of quantitation; STD, sexually
transmitted disease; ELISA, enzyme-linked immunosorbent assay; OR, odds ratio;
CI, confidence interval; ARV, antiretroviral; NASBA, nucleic acid
sequence-based amplification.
Kashuba AD,
Dyer JR, Kramer LM, Raasch RH, Eron JJ, Cohen MS. Antiretroviral-drug
concentrations in semen: implications for sexual transmission of human
immunodeficiency virus type 1. Antimicrob Agents Chemother. 1999;43:1817-1826.
Copyright 1999. American Society for Microbiology. All rights reserved.
In summary, the use of antiretroviral drugs to reduce the
concentration of HIV in genital secretions seems to be one important approach
to HIV prevention. Quinn and colleagues[140] provided a population model
demonstrating the potential benefits; they estimated that if serum viral burden
is reduced to less than 3500 copies/mL, HIV transmission will be reduced by
81.4%. Previous studies[45,46] have demonstrated that antiretroviral
therapy does indeed reduce blood and semen viral load below this threshold.
Considerably more is known about this topic in men than women
(although Hart and colleagues[141] recently demonstrated a reduction in HIV
RNA in female genital secretion in response to antiretroviral therapy). In
addition, there have been no large-scale trials to evaluate the cost-benefit
ratio of this approach. Using a mathematical model, Blower and coworkers[142] demonstrated that the benefit of
antiretroviral therapy that reduces but does not eliminate transmissibility
might be offset by increased risky sexual behavior. Compelling empirical data
are not available. Trials to demonstrate a reduced rate of transmission of HIV
by patients receiving antiretroviral therapy are limited by the need to enroll
discordant couples who must be counseled to avoid HIV transmission by all known
routes. The study of this subject in animal models has also been difficult;
nevertheless, it is clearly possible to use antiretroviral drugs to reduce the
burden of feline immunodeficiency virus in blood and semen in cats, and this
model probably holds some promise for study of lentivirus transmission (H.
Jordan, unpublished observation, 2000).
HIV Transmission and Resistance to Antiretroviral Agents
The public health considerations of the
use of antiretroviral therapy should certainly receive increasing attention.
Drug-resistant viral isolates are clearly fit enough to be transmitted
successfully,[143] and a substantial proportion of patients
with primary HIV infection have acquired virus that is resistant to 1 or more
antiretroviral agents.[144,145] The factors that might be expected to
lead to antiretroviral resistance are as follows:
- biological
(partial penetration of drugs into the genital tract)
- behavioral
(poor adherence to therapy)
- metabolic
(poor absorption of drugs or drug interactions that reduce blood plasma
concentrations, etc)
On account of 1 or more of these factors,
HIV in semen may be only partially suppressed by antiretrovirals, and hence
drug-resistant isolates are likely to be selected. Although it is standard
practice to measure viral burden in blood and to combat the evolution of
resistance aggressively, this is not the case in genital secretions. Several
small-scale studies have examined this problem. Eron and coworkers[143] have recently examined blood and semen
viral isolates from men undergoing antiretroviral therapy and demonstrated
emergence of resistance in both compartments that was not always concordant
(see below). Mayer and colleagues[146] and Byrn and associates[147] have made similar observations.
Eron and colleagues[143] studied 11 patients in whom potent
antiretroviral therapy failed to suppress HIV in blood plasma. Variants
resistant to NRTIs evolved in both blood and semen, although in these subjects
some differences in evolution of resistance variants were noted between the
blood and the semen. Interestingly, although several patients were taking
protease inhibitors and protease inhibitor resistance mutations evolved in
blood, such mutations were not detected in semen, supporting the idea that the
protease inhibitors may not achieve adequate concentrations in the genital
tract to provide selective pressure for the evolution of resistance.[143] However, other groups have now reported
evolution in semen of HIV-1 variants with resistance to certain protease inhibitors.[148] In addition, the ability of protease
inhibitors to penetrate into semen is variable.[44,137,138]
The recognition of resistant HIV isolates in blood and semen of
patients receiving antiretroviral therapy begs the question of the potential
role of resistance testing. In many but not all infectious diseases, the choice
of therapy is guided by sensitivity testing. Recent data suggest that testing
the drug sensitivity of viral isolates from the blood can benefit patient
management and response to therapy,[149] albeit at considerable cost. However,
since drug resistance may evolve independently in blood and semen, the public
health benefits of such testing are unproved.
Postexposure Prophylaxis
Antiretroviral agents could be given to
those at risk before or shortly after exposure to HIV. Work with macaques
demonstrates that pre-exposure and early postexposure prophylaxis can work
well, depending on the timing and the agent(s) chosen.[150] There is no clinical experience with
pre-exposure prophylaxis, although trials among selected commercial sex workers
are in the planning stages.
Prompt use of antiretroviral drugs after occupational exposure to
HIV in health care settings has been associated with a reduced risk of
acquiring HIV. In a single, large case-control study, Cardo and colleagues[151] compared acquisition of HIV in 33 health
care workers infected by occupational exposure compared with 665 exposed but
uninfected controls. The use of zidovudine as postexposure prophylaxis appeared
to be protective, with significantly fewer cases than controls having used
zidovudine.
Galvanized by these findings, several organizations have
recommended that subjects exposed to HIV through unprotected intercourse under
very specific circumstances might receive postexposure prophylaxis (PEP).
Specifically, both the Centers for Disease Control and Prevention and an expert
panel recommended that subjects exposed to high-risk patients, especially after
rape, should consider using antiretroviral drugs.[152] However, these authorities also recognize
the lack of data to support the efficacy of this approach, and it is unclear
whether data regarding occupational exposures can be extrapolated to other
types of exposure, where the biological mechanisms of transmission may be quite
different. Indeed, the only available data on PEP for sexual exposure come from
a limited study of the use of drugs in macaques exposed to intravaginal HIV
introduced through cell-free virus. The drug PMPA appeared to offer protection
when provided either before or even 24 hours after introduction of the virus.[150]
On the other hand, some investigators have explored the
possibility of more routine use of PEP. In a series of articles, Gerberding and
Katz[153,154] investigated the rationale for PEP after nonoccupational
exposures and tried to analyze the cost-benefit ratio for the use of such
drugs. Unfortunately, although it is possible to estimate the financial cost of
the medical intervention, it is not possible to reliably estimate the
likelihood that a random sexual partner will be infected with HIV or the likely
benefit of PEP in reducing the risk of transmission. Indeed, because the
pharmacology of antiretroviral drugs in the genital tract is only poorly
understood and because semen can persist in vaginal secretions for a very long
period, assumptions cannot be made. Investigators in San Francisco, California,
have launched a multicenter program to provide access to counseling, PEP (if
appropriate), and HIV antibody testing for individuals reporting recent unsafe
sexual or drug-injecting behaviors.[155] However, although data are being gathered
on types of risk exposure, uptake of PEP, adherence, drug adverse effects, and
longitudinal trends in risk-taking behavior, the study design does not allow assessment
of the efficacy of PEP.
Currently, it seems very unlikely that a study in the United
States could properly evaluate the potential benefit of PEP in subjects exposed
to HIV. Accordingly, to try to evaluate this approach, the Centers for Disease
Control and Prevention has recently launched a registry to assess PEP after
nonoccupational HIV exposure.
There is particular interest in the use of PEP following rape. The
French government has reportedly legislated that women who survive rape should
be offered antiretroviral prophylaxis, along with appropriate counseling
services. Benais and coworkers[156] have reported their experience in 5
emergency medicolegal units in Paris that have been in operation since June
1999. A total of 2550 victims of rape were offered triple-drug antiretroviral
therapy with stavudine, didanosine, and nelfinavir within 48 hours of the
assault, but only 100 subjects were treated. No biochemical toxicities were
recognized, but many patients discontinued therapy and 25% failed to return for
follow-up. No patient acquired HIV. Another study focused on sexual assault was
conducted in San Francisco, California, where PEP has been offered to all
victims of rape since April 1998.[157] Subjects who present within 72 hours of
potential exposure are offered a 10-day supply of coformulated
zidovudine/lamivudine (Combivir) with an additional 18 days of
therapy provided in a follow-up visit. A total of 376 patients were evaluated,
3% of whom were found to HIV-positive at baseline, and 213 subjects were
offered PEP. Only 32.4% of these subjects chose to initiate therapy, and only
12% returned to complete the course of therapy. White, college-educated males
with stable housing and subjects reporting anal penetration were the most
likely to initiate prophylaxis. A course of therapy cost $327. The authors
concluded that PEP should be offered to all victims of a rape as part of
comprehensive counseling. However, it is clear that even when offered therapy,
many patients -- even victims of rape -- choose not to accept it, and
furthermore, therapy may not be completed by those who do initiate it.
Routine use of drugs after sexual exposure is fraught with
additional concerns. First, the drugs are expensive. Second, the drugs have
substantial toxicity, and the long-term consequences of taking them are not
known. Third, one might predict that, in cases where it is unclear whether
exposure to HIV has occurred, subjects might be poorly adherent to PEP
regimens, and in the rare subject who was exposed, drug resistance might
evolve. In summary, it seems unlikely that PEP after unknown sexual exposure
will become routine public health policy in the foreseeable future.[158] Postexposure prophylaxis after high risk
or known exposure will probably become commonplace, even without definitive
supportive data.[159]
Conclusion
The recognition of HIV as the etiological
agent responsible for AIDS quickly led to a serologic test and a misplaced
belief that a preventive vaccine would soon follow. However, the search for a
vaccine has led to an explosion in knowledge about the behavior and biology of
HIV transmission by all routes. This information has informed development of
other interventions: safer sex behaviors, condoms, and better treatment of
STDs. Alternative biological interventions, including protective microbicides
and antiretroviral agents, are progressing rapidly. From this review it should
be clear that biomedical and behavioral approaches to HIV prevention are being
simultaneously developed. The biomedical interventions cannot be implemented
without the behavioral change components to support and amplify them. The tools
to prevent HIV transmission are being forged. It seems likely that their
application will slow the spread of the epidemic until curative therapy becomes
available.
References
- Royce
RA, Sena A, Cates W Jr, Cohen MS. Sexual transmission of HIV. N Engl J
Med. 1997;336:1072-1078.
- Padian
N, Marquis L, Francis DP, et al. Male-to-female transmission of human immunodeficiency
virus. JAMA. 1987;258:788-790.
- Padian
NS, Shiboski SC, Jewell NP. Female-to-male transmission of human
immunodeficiency virus. JAMA. 1991;266:1664-1667.
- Schacker
T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic
features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
- Dillon
B, Hecht FM, Swanson M, et al. Primary HIV infections associated with oral
transmission. Program and abstracts of the Seventh Conference on
Retroviruses and Opportunistic Infections; January 30-February 2, 2000;
San Francisco, Calif. Abstract 473.
- Ruprecht
RM, Baba TW, Liska V, et al. Oral transmission of primate lentiviruses. J
Infect Dis. 1999;179:S408-S412.
- Ruprecht
RM, Baba TW, Liska V, et al. Oral SIV, SHIV, and HIV type 1 infection.
AIDS Res Hum Retroviruses. 1998;14:S97-S103.
- Anderson
R, May R. Infectious Diseases of Humans: Dynamics and Control.
New York, NY: Oxford Science Publications; 1992.
- Vernazza
PL, Eron JJ, Fiscus SA, Cohen MS. Sexual transmission of HIV:
infectiousness and prevention. AIDS. 1999;13:155-166.
- Buchacz
KA, Wilkinson DA, Krowka JF, Koup RA, Padian NS. Genetic and immunological
host factors associated with susceptibility to HIV-1 infection. AIDS.
1998;12:S87-S94.
- Dyer J,
Gilliam B, Eron J, Grosso L, Cohen M, Fiscus S. Quantitation of HIV-1 RNA
in cell-free seminal plasma by NASBA amplification and Amplicor reverse
transcription-PCR: a comparative study. J Virol Methods. 1996;60:161-170.
- Vernazza
PL, Gilliam BL, Dyer J, et al. Quantification of HIV in semen: correlation
with antiviral treatment and immune status. AIDS. 1997;11:987-993.
- Iversen
AK, Larsen AR, Jensen T, et al. Distinct determinants of human
immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical
secretions. J Infect Dis. 1998;177:1214-1220.
- Hart CE,
Lennox JL, Pratt-Palmore M, et al. Correlation of human immunodeficiency
virus type 1 RNA levels in blood and the female genital tract. J Infect
Dis. 1999;179:871-882.
- Cu-Uvin
S, Caliendo AM. Cervicovaginal human immunodeficiency virus secretion and
plasma viral load in human immunodeficiency virus-seropositive women.
Obstet Gynecol. 1997;90:739-743.
- Garcia
P, Kalish L, Pitt J, et al. Maternal levels of plasma human
immunodeficiency virus type 1 RNA and the risk of perinatal transmission.
N Engl J Med. 1999;341:394-398.
- Mofenson
L, Lambert J, Stiehm E, et al, for the Pediatric AIDS Clinical Trials
Group Study 185 Team. Risk factors for perinatal transmission of human
immunodeficiency virus type 1 in women treated with zidovudine. N Engl J
Med. 1999;341:385-393.
- St Louis
ME, Kamenga M, Brown C, et al. Risk for perinatal HIV-1 transmission
according to maternal immunologic, virologic, and placental factors. JAMA.
1993;269:2853-2859.
- Busch M,
Operskalski E, Mosley J, et al, for the Transfusion Safety Study Group.
Factors influencing human immunodeficiency virus type 1 transmission by
blood transfusion. J Infect Dis. 1996;174:26-33.
- Lee TH,
Sakahara N, Fiebig E, Busch MP, O'Brien TR, Herman SA. Correlation of
HIV-1 RNA levels in plasma and heterosexual transmission of HIV-1 from
infected transfusion recipients. J Acquir Immune Defic Syndr Hum
Retrovirol. 1996;12:427-428.
- Ragni M,
Faruki H, Kingsley L. Heterosexual HIV-1 transmission and viral load in
hemophilic patients. J Acquir Immune Defic Syndr Hum Retrovirol.
1998;17:42-45.
- Quinn
TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual
transmission of human immunodeficiency virus type 1. N Engl J Med.
2000;342:921-929.
- Gray RH,
Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per
coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai,
Uganda. Lancet. 2001;357:1149-1153.
- Coombs
RW, Speck CE, Hughes JP, et al. Association between culturable human
immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in
semen and blood: evidence for compartmentalization of HIV-1 between semen
and blood. J Infect Dis. 1998;177:320-330.
- Dyer JR,
Gilliam BL, Eron JJ Jr, Cohen MS, Fiscus SA, Vernazza PL. Shedding of
HIV-1 in semen during primary infection. AIDS. 1997;11:543-545.
- Fleming
DT, Wasserheit JN. From epidemiological synergy to public health policy
and practice: the contribution of other sexually transmitted diseases to
sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3-17.
- Vernazza
P, Perrin L, Vora S, et al. Increased seminal shedding of HIV during
primary infection augments the need for earlier diagnosis and prevention.
Program and abstracts of the 7th Conference on Retroviruses and
Opportunistic Infections; January 30-February 2, 2000; San Francisco,
Calif. Abstract 564.
- Pilcher
CD, Shugars DC, Fiscus SA, et al. In subjects with primary HIV infection,
high levels of HIV RNA are present in oral fluids, genital secretions,
peripheral blood, and CNS and are rapidly reduced with combination
antiretroviral therapy. Program and abstracts of the 7th Conference on
Retroviruses and Opportunistic Infections; January 30-February 2, 2000;
San Francisco, Calif. Abstract 556.
- Jacquez
JA, Koopman JS, Simon CP, Longini IM Jr. Role of the primary infection in
epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr.
1994;7:1169-1184.
- Cates W
Jr, Chesney MA, Cohen MS. Primary HIV infection--a public health
opportunity. Am J Public Health. 1997;87:1928-1930.
- Pilcher
CD, Vernazza P, Battegay M, et al. Sexual transmission can precede
symptoms in primary HIV-1 infection. Program and abstracts of the 8th
Conference on Retroviruses and Opportunistic Infections; February 4-8,
2001; Chicago, Illinois. Abstract 411.
- Yerly S,
Race E, Vora S, et al. HIV drug resistance and molecular epidemiology in
patients with primary HIV infection. Program and abstracts of the 8th
Conference on Retroviruses and Opportunistic Infections; February 4-8,
2001; Chicago, Illinois. Abstract 754.
- Chakraborty
H, Helms RW, Sen PK, et al. Comparison of HIV transmission probability
between US + Switzerland and Malawi. Program and abstracts of the 8th
Conference on Retroviruses and Opportunistic Infections; February 4-8,
2001; Chicago, Illinois. Abstract 222.
- Chakraborty
H, Sen PK, Helms RW, et al. Viral burden in genital secretions determines
male-to-female sexual transmission of HIV-1: a probabilistic empiric
model. AIDS. 2001;15:621-627.
- Zhu T,
Wang N, Carr A, et al. Genetic characterization of human immunodeficiency
virus type 1 in blood and genital secretions: evidence for viral
compartmentalization and selection during sexual transmission. J Virol.
1996;70:3098-3107.
- DePasquale
M, Rosenberg E, Quayle A, et al. Primary HIV infection: in vivo fitness of
pre-therapy resistant mutants and potential for secondary spread of HIV
from semen. Antiviral Ther. 1999;4(suppl 1):89.
- Zhu T,
Mo H, Wang N, et al. Genotypic and phenotypic characterization of HIV-1
patients with primary infection. Science. 1993;261:1179-1181.
- Zhang
LQ, MacKenzie P, Cleland A, Holmes EC, Brown AJ, Simmonds P. Selection for
specific sequences in the external envelope protein of human
immunodeficiency virus type 1 upon primary infection. J Virol.
1993;67:3345-3356.
- Littman
DR. Chemokine receptors: keys to AIDS pathogenesis? Cell. 1998;93:677-680.
- Patterson
BK, Landay A, Andersson J, et al. Repertoire of chemokine receptor
expression in the female genital tract: implications for human
immunodeficiency virus transmission. Am J Pathol. 1998;153:481-490.
- Patterson
BK, Czerniewski MA, Pottage J, Agnoli M, Kessler H, Landay A. Monitoring
HIV-1 treatment in immune-cell subsets with ultrasensitive
fluorescence-in-situ hybridisation. Lancet. 1999;353:211-212.
- Patterson
BK, Czerniewski M, Andersson J, et al. Regulation of CCR5 and CXCR4
expression by type 1 and type 2 cytokines: CCR5 expression is
downregulated by IL-10 in CD4-positive lymphocytes. J Appl Biomaterials
(Orlando). 1999;91:254-262.
- Sodora
DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing
infectivity and hormonal influences in macaques inoculated with cell-free
and cell-associated viral stocks. AIDS Res Hum Retroviruses.
1998;14:S119-S123.
- Kashuba
A, Dyer J, Kramer L, Raasch R, Eron J, Cohen M. Antiretroviral drug
concentrations in semen: implications for sexual transmission of human
immunodeficiency virus type 1. Antimicrob Agents Chemother.
1999;43:1817-1826.
- Vernazza
P, Troiani L, Flepp M, et al. Potent antiretroviral treatment of
HIV-infection results in suppression of the seminal shedding of HIV. AIDS.
2000;14(2):117-121.
- Zhang H,
Dornadula G, Beumont M, et al. Human immunodeficiency virus type 1 in the
semen of men receiving highly active antiretroviral therapy. N Engl J Med.
1998;339:1803-1809.
- Ou CY,
Takebe Y, Weniger BG, et al. Independent introduction of two major HIV-1
genotypes into distinct high-risk populations in Thailand. Lancet.
1993;341:1171-1174.
- Kunanusont
C, Foy HM, Kreiss JK, et al. HIV-1 subtypes and male-to-female transmission
in Thailand. Lancet. 1995;345:1078-1083.
- Soto-Ramirez
LE, Renjifo B, McLane MF, et al. HIV-1 Langerhans' cell tropism associated
with heterosexual transmission of HIV. Science. 1996;271:1291-1293.
- Pope M,
Ho DD, Moore JP, Weber J, Dittmar MT, Weiss RA. Different subtypes of
HIV-1 and cutaneous dendritic cells. Science. 1997;278:786-788.
- Pope M,
Frankel SS, Mascola JR, et al. Human immunodeficiency virus type 1 strains
of subtypes B and E replicate in cutaneous dendritic cell-T-cell mixtures
without displaying subtype-specific tropism. J Virol. 1997;71:8001-8007.
- Duerr A,
Hsia J, Nicolosi A, et al. Probability of male-to-female HIV transmission
among couples with HIV subtype E and B. Program and abstracts of the XIII
International AIDS Conference; July 9-14, 2000; Durban, South Africa.
Abstract WePpC1319.
- Ping
L-H, Nelson J, Hoffman I, et al. Characterization of V3 sequence
heterogeneity in subtype C human immunodeficiency virus type 1 isolates
from Malawi: underrepresentation of X4 variants. J Virol. 1999;73:6271-6281.
- Bjorndal
A, Sonnerborg A, Tscherning C, Albert J, Fenyo EM. Phenotypic
characteristics of human immunodeficiency virus type 1 subtype C isolates
of Ethiopian AIDS patients. AIDS Res Hum Retroviruses. 1999;15:647-653.
- Koot M,
Keet I, Vos A, et al. Prognostic value of HIV-1 syncytium-inducing
phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann
Intern Med. 1993;118:681-688.
- Bozzette
SA, McCutchan JA, Spector SA, Wright B, Richman DD. A cross-sectional
comparison of persons with syncytium- and non-syncytium-inducing human
immunodeficiency virus. J Infect Dis. 1993;168:1374-1379.
- Schuitemaker
H, Koot M, Kootstra NA, et al. Biological phenotype of human
immunodeficiency virus type 1 clones at different stages of infection: progression
of disease is associated with a shift from monocytotropic to T-cell-tropic
virus population. J Virol. 1992;66:1354-1360.
- Tien PC,
Chiu T, Latif A, et al. Primary subtype C HIV-1 infection in Harare,
Zimbabwe. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:147-153.
- Hoffman
TL, Doms RW. Chemokines and coreceptors in HIV/SIV-host interactions.
AIDS. 1998;12:S17-S26.
- Samson
M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian
individuals bearing mutant alleles of the CCR-5 chemokine receptor gene.
Nature. 1996;382:722-725.
- Michael
NL, Chang G, Louie LG, et al. The role of viral phenotype and CCR-5 gene
defects in HIV-1 transmission and disease progression. Nature Med. 1997;3:338-340.
- Hoffman
TL, MacGregor RR, Burger H, Mick R, Doms RW, Collman RG. CCR5 genotypes in
sexually active couples discordant for human immunodeficiency virus type 1
infection status. J Infect Dis. 1997;176:1093-1096.
- Fowke
KR, Nagelkerke NJ, Kimani J, et al. Resistance to HIV-1 infection among
persistently seronegative prostitutes in Nairobi, Kenya. Lancet.
1996;348:1347-1351.
- Taha TE,
Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of
vaginal flora: association with increased acquisition of HIV. AIDS.
1998;12:1699-1706.
- Sewankambo
N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal
vaginal flora morphology and bacterial vaginosis. Lancet.
1997;350:546-550.
- Mazzoli
S, Trabattoni D, Lo Caputo S, et al. HIV-specific mucosal and cellular
immunity in HIV-seronegative partners of HIV-seropositive individuals.
Nature Med. 1997;3:1250-1257.
- Kaul R,
Trabattoni D, Bwayo JJ, et al. HIV-1-specific mucosal IgA in a cohort of
HIV-1-resistant Kenyan sex workers. AIDS. 1999;13:23-29.
- Rowland-Jones
SL, Dong T, Fowke KR, et al. Cytotoxic T cell responses to multiple
conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin
Invest. 1998;102:1758-1765.
- Pinto
LA, Landay AL, Berzofsky JA, Kessler HA, Shearer GM. Immune response to
human immunodeficiency virus (HIV) in healthcare workers occupationally
exposed to HIV-contaminated blood. Am J Med. 1997;102:21-24.
- Halperin
DT, Bailey RC. Viewpoint: male circumcision and HIV infection: 10 years
and counting. Lancet. 1999;354:1813-1815.
- Van Howe
RS. Circumcision and HIV infection: review of the literature and
meta-analysis. Intl J STD AIDS. 1999;10:8-16.
- Gwanzura
L, Machekano R, Bassett M, et al. HIV-1/HSV-2 co-infection and
sero-concordance among heterosexual couples in Harare, Zimbabwe. Program
and abstracts of the XIII International AIDS Conference; July 9-14, 2000;
Durban, South Africa. Abstract ThOrC726.
- Cohen
MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in
semen after treatment of urethritis: implications for prevention of sexual
transmission of HIV-1: AIDSCAP Malawi Research Group. Lancet.
1997;349:1868-1873.
- Levine
WC, Pope V, Bhoomkar A, et al. Increase in endocervical CD4 lymphocytes
among women with nonulcerative sexually transmitted diseases. J Infect
Dis. 1998;177:167-174.
- Dyer J,
Eron J, Hoffman I, et al. Association of CD4 cell depletion and elevated
blood and seminal plasma human immunodeficiency virus type 1 (HIV-1) RNA
concentrations with genital ulcer disease in HIV-1-infected men in Malawi.
J Infect Dis. 1998;177:224-227.
- Anderson
DJ, Politch JA, Tucker LD, et al. Quantitation of mediators of
inflammation and immunity in genital tract secretions and their relevance
to HIV type 1 transmission. AIDS Res Hum Retroviruses. 1998;14:S43-S49.
- Spear
GT, Sha BE, Saarloos MN, et al. Chemokines are present in the genital
tract of HIV-seropositive and HIV-seronegative women: correlation with
other immune mediators. J Acquir Immune Defic Syndr. 1998;18:454-459.
- Al-Harthi
L, Spear GT, Hashemi FB, Landay A, Sha BE, Roebuck KA. A human
immunodeficiency virus (HIV)-inducing factor from the female genital tract
activates HIV-1 gene expression through the kappaB enhancer. J Infect Dis.
1998;178:1343-1351.
- Kreiss
J, Willerford DM, Hensel M, et al. Association between cervical
inflammation and cervical shedding of human immunodeficiency virus DNA. J
Infect Dis. 1994;170:1597-1601.
- Ghys PD,
Fransen K, Diallo MO, et al. The associations between cervicovaginal HIV
shedding, sexually transmitted diseases and immunosuppression in female
sex workers in Abidjan, Cote d'Ivoire. AIDS. 1997;11:F85-F93.
- Mostad
SB, Jackson S, Overbaugh J, et al. Cervical and vaginal shedding of human
immunodeficiency virus type 1-infected cells throughout the menstrual cycle.
J Infect Dis. 1998;178:983-991.
- Martin
HL Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually
transmitted diseases, and risk of heterosexual transmission of human
immunodeficiency virus type 1. J Infect Dis. 1998;178:1053-1059.
- Wawer
MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted
diseases for AIDS prevention in Uganda: a randomised community trial:
Rakai Project Study Group. Lancet. 1999;353:525-535.
- Smith S,
Charkraborty P, Baskin G, Marx P. Estrogen protects against vaginal
transmission of SIV. J Infect Dis. 2000;182:708-715.
- Sweat
MD, Denison JA. Reducing HIV incidence in developing countries with
structural and environmental interventions. AIDS. 1995;9:S251-S257.
- De Zoysa
I, Phillips KA, Kamenga MC, et al. Role of HIV counseling and testing in
changing risk behavior in developing countries. AIDS. 1995;9:S95-S101.
- Lamptey
PR, Price JE. Social marketing sexually transmitted disease and HIV
prevention: a consumer-centered approach to achieving behaviour change.
AIDS. 1998;12:S1-S9.
- Lamptey
PR, Kamenga MC, Weir SS. Prevention of sexual transmission of HIV in
sub-Saharan Africa: lessons learned. AIDS. 1997;11:S63-S77.
- Cohen M,
Dallabetta G, Holmes K, Cates W. Global Prevention of HIV
Transmission: The Medical Management of AIDS. Philadelphia, Pa:
WB Saunders Co; 1999.
- Kahn JG.
The cost-effectiveness of HIV prevention targeting: how much more bang for
the buck? Am J Public Health. 1996;86:1709-1712.
- Kotler
P. Social Marketing: Changing Public Behavior by Persuasion.
New York, NY: Free Press; 1989.
- Kamb ML,
Fishbein M, Douglas JM Jr, et al. Efficacy of risk-reduction counseling to
prevent human immunodeficiency virus and sexually transmitted diseases: a
randomized controlled trial: Project RESPECT Study Group. JAMA.
1998;280:1161-1167.
- Shain R,
Piper J, Newton E, et al. A randomized, controlled trial of a behavioral
intervention to prevent sexually transmitted disease among minority women.
N Engl J Med. 1999;340:93-100.
- The
National Institute of Mental Health Multisite HIV Prevention Trial Group.
The NIMH Multisite HIV Prevention Trial: reducing HIV sexual risk
behavior. Science. 1998;280:1889-1894.
- Kelly
JA, St. Lawrence JS, Stevenson LY, et al. Community AIDS/HIV risk
reduction: the effects of endorsements by popular people in three cities.
Am J Public Health. 1992;82:1483-1489.
- Des
Jarlais DC, Padian NS, Winkelstein W Jr. Targeted HIV-prevention programs.
N Engl J Med. 1994;331:1451-1453.
- Ruiz MS,
Gable AR, Kaplan EH, Stoto MA, Fineberg HV, Trussel J, eds. No Time To
Lose. Getting More from HIV Prevention. 2000. Institute of Medicine.
National Academy Press, Washington, DC.
- Cohen
MS, Henderson GE, Aiello P, Zheng H. Successful eradication of sexually
transmitted diseases in the People's Republic of China: implications for
the 21st century. J Infect Dis. 1996;174:S223-S229.
- Adimora
AA, Schoenbach VJ, Martinson FE, Donaldson KH, Fullilove RE, Aral SO.
Social context of sexual relationships among rural African Americans. Sex
Transm Dis. 2001;28:69-76.
- Stratton
P, Alexander NJ. Prevention of sexually transmitted infections: physical
and chemical barrier methods. Infect Dis Clin North Am. 1993;7:841-859.
- Cates W.
How much do condoms protect against sexually transmitted diseases? IPPF
Med Bull. 1997;31:2-3.
- de
Vincenzi I. A longitudinal study of human immunodeficiency virus
transmission by heterosexual partners: European Study Group on
Heterosexual Transmission of HIV. N Engl J Med. 1994;331:341-346.
- Deschamps
MM, Pape JW, Hafner A, Johnson WD Jr. Heterosexual transmission of HIV in
Haiti. Ann Intern Med. 1996;125:324-330.
- Nelson
KE, Celentano DD, Eiumtrakol S, et al. Changes in sexual behavior and a
decline in HIV infection among young men in Thailand. N Engl J Med. 1996;335:297-303.
- Albert
AE, Warner DL, Hatcher RA. Facilitating condom use with clients during
commercial sex in Nevada's legal brothels. Am J Public Health.
1998;88:643-646.
- Crabbe
F, Tchupo JP, Manchester T, et al. Prepackaged therapy for urethritis: the
"MSTOP" experience in Cameroon. Sex Transm Infect.
1998;74:249-252.
- Musaba
E, Morrison CS, Sunkutu MR, Wong EL. Long-term use of the female condom
among couples at high risk of human immunodeficiency virus infection in
Zambia. Sex Transm Dis. 1998;25:260-264.
- Chesson
HW, Pinkerton SD, Irwin KL, Rein D, Kassler WJ. New HIV cases attributable
to syphilis in the USA: estimates from a simplified transmission model.
AIDS. 1999;13:1387-1396.
- Adimora
AA, Sparling PF, Cohen MS. Vaccines for classic sexually transmitted
diseases. Infect Dis Clin North Am. 1994;8:859-876.
- Blower
SM, McLean AR. Prophylactic vaccines, risk behavior change, and the
probability of eradicating HIV in San Francisco. Science.
1994;265:1451-1454.
- Delwart
EL, Mullins JI, Gupta P, et al. Human immunodeficiency virus type 1
populations in blood and semen. J Virol. 1998;72:617-623.
- AIDS
Vaccine Evaluation Group. The AVEG Information Handbook.
Data Coordinating and Analysis Center; May 2000. Available at:
http://scharp.org/public/redbook/contents.htm
- Louv WC,
Austin H, Alexander WJ, Stagno S, Cheeks J. A clinical trial of
nonoxynol-9 for preventing gonococcal and chlamydial infections. J Infect
Dis. 1988;158:518-523.
- Barbone
F, Austin H, Louv WC, Alexander WJ. A follow-up study of methods of
contraception, sexual activity, and rates of trichomoniasis, candidiasis,
and bacterial vaginosis. Am J Obstet Gynecol. 1990;163:510-514.
- Kreiss
J, Ngugi E, Holmes K, et al. Efficacy of nonoxynol 9 contraceptive sponge
use in preventing heterosexual acquisition of HIV in Nairobi prostitutes.
JAMA. 1992;268:477-482.
- Roddy
RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of
nonoxynol 9 film to reduce male-to-female transmission of sexually
transmitted diseases. N Engl J Med. 1998;339:504-510.
- Mostad
SB, Overbaugh J, DeVange DM, et al. Hormonal contraception, vitamin A
deficiency, and other risk factors for shedding of HIV-1 infected cells
from the cervix and vagina. Lancet. 1997;350:922-927.
- van
Damme L. Roundtable: Prevention of HIV. Program and abstracts of the XIII
International AIDS Conference; July 9-14, 2000; Durban, South Africa.
Session WeOr62.
- Hoffman
I, Taha TE, Martinson F, et al. Adverse health events occurring during an
N-9 gel pilot study: Malawi. Program and abstracts of the XIII
International AIDS Conference; July 9-14, 2000; Durban, South Africa.
Abstract TuPpC1171.
- John GC,
Nduati RW, Mbori-Ngacha D, et al. Genital shedding of human immunodeficiency
virus type 1 DNA during pregnancy: association with immunosuppression,
abnormal cervical or vaginal discharge, and severe vitamin A deficiency. J
Infect Dis. 1997;175:57-62.
- Moss GB,
Overbaugh J, Welch M, et al. Human immunodeficiency virus DNA in urethral
secretions in men: association with gonococcal urethritis and CD4 cell
depletion. J Infect Dis. 1995;172:1469-1474.
- Grosskurth
H, Mosha F, Todd J, et al. Impact of improved treatment of sexually
transmitted diseases on HIV infection in rural Tanzania: randomised
controlled trial. Lancet. 1995;346:530-536.
- Gilson
L, Mkanje R, Grosskurth H, et al. Cost-effectiveness of improved treatment
services for sexually transmitted diseases in preventing HIV-1 infection
in Mwanza Region, Tanzania. Lancet. 1997;350:1805-1809.
- Steen R,
Dallabetta G. The use of epidemiologic mass treatment and syndrome
management for sexually transmitted disease control. Sex Transm Dis.
1999;26:S12-20; discussion S21-S2.
- Hitchcock
P, Fransen L. Preventing HIV infection: lessons from Mwanza and Rakai.
Lancet. 1999;353:513-515.
- Pudney
J, Anderson DJ. Immunobiology of the human penile urethra. Am J Pathol.
1995;147:155-165.
- Bailey
R. Male circumcision as an effective HIV prevention strategy: current
evidence. Program and abstracts of the 8th Conference on Retroviruses and
Opportunistic Infections; February 4-8, 2001; Chicago, Illinois. Abstract
S22.
- Buve A,
Auvert B, Lagare E, Kahindo M, Hayes R, Carael M. Male circumcision and
HIV spread in sub-Saharan Africa. Program and abstracts of the XIII
International AIDS Conference; July 9-14, 2000; Durban, South Africa.
Abstract MoOrC192.
- Gray R,
Kiwanuka N, Quinn TC, et al. Male circumcision and HIV acquisition and
transmission: cohort studies in Rakai, Uganda. Rakai Project Team. AIDS.
2000;14:2371-2381.
- Taljaard
R, Taljaard D, Auvert B, Neilssen G. Cutting it fine: male circumcision
practises and the transmission of STDs in Carletonville. Program and
abstracts of the XIII International AIDS Conference; July 9-14, 2000;
Durban, South Africa. Abstract MoOrC195.
- Bailey
R, Muga R, Poulussen R. Trial intervention introducing male circumcision
to reduce HIV/STD infections in Nyanza province, Kenya: baseline results.
Program and abstracts of the XIII International AIDS Conference; July 9-14,
2000; Durban, South Africa. Abstract MoOrC196.
- Cohen
MS. Preventing sexual transmission of HIV: new ideas from sub-Saharan
Africa. N Engl J Med. 2000;342:970-972.
- Musicco
M, Lazzarin A, Nicolosi A, et al. Antiretroviral treatment of men infected
with human immunodeficiency virus type 1 reduces the incidence of
heterosexual transmission: Italian Study Group on HIV Heterosexual
Transmission. Arch Intern Med. 1994;154:1971-1976.
- Henry K,
Chinnock BJ, Quinn RP, Fletcher CV, de Miranda P, Balfour H Jr. Concurrent
zidovudine levels in semen and serum determined by radioimmunoassay in
patients with AIDS or AIDS-related complex. JAMA. 1988;259:3023-3026.
- Pereira
A, Kashuba A, Fiscus S, et al. Nucleoside analogues achieve high
concentrations in seminal plasma: relationship between drug concentrations
and viral burden. J Infect Dis. In press.
- Taylor
S, Back D, Workman J, et al. Poor penetration of the male genital tract by
HIV-1 protease inhibitors. AIDS. 1999;13:859-860.
- van
Praag R, Weverling G, Portegies P, et al. Enhanced penetration of
indinavir in cerebrospinal fluid and semen after the addition of low-dose
ritonavir. AIDS. 2000;14:1187-1194.
- Pereira
A, Eron J, Dunn J, et al. Analysis of amprenavir (APV)/3TC/ZDV in blood
and seminal plasma: penetration of APV into the male genital tract.
Program and abstracts of the Seventh Conference on Retroviruses and
Opportunistic Infections; January 30-February 2, 2000; San Francisco,
Calif. Abstract 317.
- Kim J,
Gotzkowsky K, Eron J, et al. Penetration of efavirenz (EFV) into the male
genital tract (GT) as measured by seminal plasma (SP) to blood plasma (BP)
concentration (CONC) and AUC24h ratios. Program and abstracts of the 40th
Interscience Conference on Antimicrobial Agents and Chemotherapy; Toronto,
Ontario, Canada; September 17-20, 2000. Abstract 1171.
- Quinn T,
Gray R, Sewankambo N, et al. Therapeutic reductions of HIV viral load to
prevent HIV transmission: data from HIV discordant couples; Rakai, Uganda.
Program and abstracts of the XIII International AIDS Conference; July
9-14, 2000; Durban, South Africa. Abstract TuPeC3391.
- Hart CE,
Lennox JL, Pratt-Palmore M, et al. Correlation of human immunodeficiency
virus type 1 RNA levels in blood and the female genital tract. J Infect
Dis. 1999;179:871-882.
- Blower
SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and
antiretroviral therapy in San Francisco. Science. 2000;287:650-654.
- Eron J,
Vernazza P, Johnston D, et al. Resistance of HIV-1 to antiretroviral
agents in blood and seminal plasma: implications for transmission. AIDS.
1998;12:F181-F189.
- Little
SJ, Daar ES, D'Aquila RT, et al. Reduced antiretroviral drug
susceptibility among patients with primary HIV infection. JAMA.
1999;282:1142-1149.
- Brodine
SK, Shaffer RA, Starkey MJ, et al. Drug resistance patterns, genetic
subtypes, clinical features, and risk factors in military personnel with
HIV-1 seroconversion. Ann Intern Med. 1999;131:502-506.
- Mayer K,
Boswell S, Goldstein R, et al. Persistence of human immunodeficiency virus
in semen after adding indinavir to combination antiretroviral therapy.
Clin Infect Dis. 1999;28:1252-1259.
- Byrn R,
Zhang D, Eyre R, McGowan K, AA K. HIV-1 in semen: an isolated virus
reservoir. Lancet. 1997;350:1141.
- DePasquale
M, Kartsonia N, Martinez-Picado J, et al. Selection of protease resistance
mutations in semen. Program and abstracts of the 6th Conference on
Retroviruses and Opportunistic Infections; January 31-February 4, 1999;
Chicago, Ill. Abstract 11.
- Durant
J, Clevenbergh P, Halfon P, et al. Drug-resistance genotyping in HIV-1
therapy: the VIRADAPT randomised controlled trial. Lancet.
1999;353:2195-2199.
- Tsai CC,
Follis KE, Sabo A, et al. Prevention of SIV infection in macaques by
(R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197-1199.
- Cardo
DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV
seroconversion in health care workers after percutaneous exposure: Centers
for Disease Control and Prevention Needlestick Surveillance Group. N Engl
J Med. 1997;337:1485-1490.
- Centers
for Disease Control and Prevention. Guidelines for treatment of sexually
transmitted diseases. MMWR Morb Mortal Wkly Rep. 1998;47:109-111.
- Katz MH,
Gerberding JL. Postexposure treatment of people exposed to the human
immunodeficiency virus through sexual contact or injection-drug use. N
Engl J Med. 1997;336:1097-100.
- Katz MH,
Gerberding JL. The care of persons with recent sexual exposure to HIV. Ann
Intern Med. 1998;128:306-312.
- Kahn JO,
Martin JN, Roland ME, et al. Feasibility of postexposure prophylaxis (PEP)
against human immunodeficiency virus infection after sexual or injection
drug use exposure: San Francisco PEP Study. J Infect Dis.
2001;183:707-714.
- Benais
J-P, Miara A, Brion S, et al. Treatment of sexual assault: a
multicentrique study in emergency medico-legal units in the Paris region.
Program and abstracts of the XIII International AIDS Conference; July
9-14, 2000; Durban, South Africa. Abstract TuOrC314.
- Myles
JE, Hirozawa A, Katz MH, et al. Postexposure prophylaxis for HIV after
sexual assault. JAMA. 2000;284:1516-1517.
- Lurie P,
Miller S, Hecht F, Chesney M, Lo B. Postexposure prophylaxis after
nonoccupational HIV exposure: clinical, ethical and policy considerations.
JAMA. 1998;280:1769-1773.
- France
D. Emergency treatment to stop AIDS virus. New York Times. October 19,
1999: D7.
Appendix: Talking About Safer Sex With Your Patients
(Reproduced with permission from a
brochure written by Marshall Miller, Kenneth Mayer, MD, and Harvey J. Makadon,
MD, published by The Fenway Institute/Fenway Community Health, 7 Haviland
Street, Boston, MA 02115. Copyright 1999. The brochure was made possible
through a grant from the Kaiser Family Foundation, Putting HIV Prevention Into
Clinical Practice, Harvey J. Makadon, MD, Principal Investigator.)
Think Prevention
Health care providers can play an
important role in HIV prevention. Many people consider their doctor the person
they most trust to give advice about the issues around health and sexuality.
Some health care providers find that the most useful approach to talking about
safer sex with their patients is to include it in a broader discussion about
all aspects of healthier living. Just as a physician may discuss with his or
her patient the importance of exercise or seat belts, or the health benefits of
quitting smoking, he or she may discuss ways the patient can reduce the risk
for HIV infection.
Communicate About Sexuality
In our culture, sexuality is rarely talked
about in honest, open terms. Given this, few people have experience asking and
responding to questions about sex. One way to overcome this barrier is to
assess and then speak about sexuality in terms that are familiar and
comfortable for the patient. These terms may vary based on the age, race,
gender, sexual orientation, and other identities of the patient. If you develop
a communication style that works for you both, it will enable you to ask the
questions and get the answers you need to provide your patients with
appropriate education, support, and referral. Workshops and trainings on this
issue are offered at conferences and other locations throughout the country.
Build Trust
People at risk for HIV are diverse; many
have a wide range of sexual experiences. Some may have experienced prejudice or
discrimination because of their sexuality. In order to be an effective HIV
educator for your patients, it is important to earn their trust. One way to do
this is to have brochures, posters, pamphlets and other information in your
office that are welcoming to people of all sexual orientations. This serves as
positive reinforcement for patients that they will be respected for who they
are. Another means of building trust is to not make any assumptions about the
type or amount of sex a person is having. Just because a person is married, for
example, does not mean that he or she is monogamous or heterosexual. Effective
trust-building results in a patient feeling comfortable talking about his or
her risks around HIV regardless of sexual orientation or past sexual
experience.
Communicate the Risks
Patients may have questions about the risk
of being infected with HIV as a result of engaging in particular sexual
activities. For example, a patient might ask, "How risky is oral
sex?". Questions like this provide a good opportunity to engage patients
in a discussion about what risks they are taking and how to reduce their
overall risk for HIV. One way of responding to the oral sex question above is
to ask the patient "Riskier than what?". Answering this question
helps the patient probe deeper into how they understand the risk of oral sex in
the context of their own lives. Oral sex is safer than unprotected vaginal or
anal sex
Recognize the Links
When counseling patients about safer sex,
it is important to help patients make connections between their sexual
risk-taking and other issues in their lives. Substance use, for example, can
put a person at higher risk for HIV. A client concerned about his or her unsafe
sex while under the influence of alcohol or other drugs, would need counseling
about substance use as well as about safer sex. Other issues, including
domestic violence, sex for drugs or money, and sexual assault are opportunities
for counseling, support, and referral.
The Biology of Safer Sex
HIV is present in semen and cervicovaginal
secretions, both as cell-free and cell-associated virus. Genital tract
secretions may contain millions of particles of HIV, especially in individuals
who are acutely infected, who have advanced disease, and who are not receiving
antiretroviral therapy. HIV inhabits white blood cells, particularly CD4
lymphocytes, and monocyte-macrophages In some individuals who are asymptomatic,
there may be more than a million of these white blood cells in genital
secretions. Other sexually transmitted diseases, including gonorrhea, chlamydia,
trichomonas, and herpes may increase the number of genital tract white cells
and may make HIV infected people more infectious to their partners. Thus,
people with HIV infection may range in their level of infectiousness to
potential partners.
The Impact of Antiretroviral Therapy
Antiretroviral therapy usually decreases
the amount of HIV in the genital tract as well as the blood. There are
individuals, however, who have been shown to have undetectable levels of virus
in the blood, and yet have virus found in the genital tract, Therefore
individuals cannot assume that because the virus is suppressed in the blood due
to therapy, they are not potentially infectious to their partners. There are
individuals who have received antiretroviral therapy and have transmitted
multi-drug resistant HIV to their partners. Thus, although people taking
combination antiretroviral therapy may be less likely to be infectious, they
need to be counseled that for any given sex act, they are potentially
infectious, and thus should practice safer sex.
Susceptibility May Vary
Just as the level of infectiousness of HIV
may vary, the level of susceptibility to HIV may vary between different groups
of people, and the same person has different levels at separate times. Sexually
transmitted diseases in HIV uninfected people may enhance susceptibility,
either by causing ulcerations in which the mucous members are exposed (eg,
herpes), or the enhanced inflammation of infections like gonorrhea or
chlamydia, may result in more target cells for HIV to infect. In addition,
genital infections result in the production of cytokines, which increase the
ability of HIV to multiply at the site of sexual contact. Some individuals may
be genetically more resistant to HIV because they lack or have decreased
amounts of some of the receptors to which HIV binds in order to enter cells.
Other factors that may influence the efficiency of HIV transmission include the
absence of circumcision and cervical ectopy. Both the human foreskin and the
endocervix contain cells that have increased number of receptors to bind HIV.
Hormonal contraception may also increase HIV susceptibility. Sexual practices
that increase trauma and/or inflammation in the genital tract, like certain
douching products, may also increase risk for HIV transmission.
Sexual Practice and Risk
Not all sexual practices are equally
likely to result in HIV transmission. The most efficient ways are through the
rectal mucous membranes, or the cervical vaginal mucosa. These tissues seem to
have more receptors to bind HIV, and in the case of the rectal mucous
membranes, the tissue is more easily traumatized, leading to more easy access
for HIV transmission. Because cervicovaginal secretions contain less HIV on the
average than semen, and because the amount of exposed tissue of the male
urethra is limited for HIV binding, it is more efficient for men to transmit
HIV to women through heterosexual contact than vice-versa. Likewise, it is
harder for an insertive partner to acquire HIV through anal intercourse than a
receptive partner. As mentioned above, there are many factors that may
influence the amount of virus in genital tract secretions and the
susceptibility of individuals at risk for HIV to acquire HIV. Therefore, it is
impossible to give precise numbers for the individual risks of each sexual act
between an infected person and an uninfected person. It is clear from many
epidemiological and biological studies, that most HIV transmission between men
who have sex with men occurs via unprotected anal intercourse, either to the
insertive or receptive partner; and for heterosexuals, most of the contact is
through unprotected penile-vaginal intercourse. Well-documented cases of HIV
transmission through oral exposure to semen or cervicovaginal secretions have
been documented so that fellatio and cunnilingus cannot be considered to be
safe practices. However, the relative inefficiency of HIV transmission in the
upper digestive system suggests that the likelihood of HIV transmission by the
oral route is several logs less likely than penile-vaginal or penile-anal
intercourse. For these higher risk practices, the per contact risk of HIV
acquisition, ranges from less than 1/1000 to more than 1/10. There is wide
biological variability for each behavior, in terms of the amount of risk for
each act.
Other sexual practices are substantially less likely to transmit
HIV. For example, although HIV has been shown to be present in minute
quantities in pre-ejaculate, there are documented reports that suggest exposure
to pre-ejaculate has resulted in transmission. Other body fluids such as saliva
have been shown to contain substances that inhibit HIV transmission, so in the
absence of visible blood, there has been no incidence of HIV transmission by
kissing. HIV can be transmitted via blood contact, and thus shared sharp
objects, piercing, shared sex toys, etc have the potential to transmit HIV.
Intimate skin contact, mutual masturbation, and other practices, which do not
result in genital secretions coming directly into contact with mucus membranes
or abraded skin have not been documented to transmit HIV (ie, the intact skin
is a good barrier to HIV transmission). Other practices may not transmit HIV,
but may result in other health problems. For example, although anal-oral contact
without blood present has not been shown to result in HIV transmission, it can
result in the transmission of enteric parasites, and serious bacterial
infections, like salmonella. Practices involving other bodily fluids may
transmit STDs as well, and need to be evaluated on a case-by-case basis.
Author Affiliations and Disclosures
Myron S.
Cohen, MD
|
Professor of Medicine, Microbiology and
Immunology, University of North Carolina at Chapel Hill School of Medicine;
Chief, Division of Infectious Diseases, UNC Hospitals, Chapel Hill, North
Carolina |
Joseph J.
Eron, MD
|
Associate Professor of Medicine,
Division of Infectious Diseases, University of North Carolina at Chapel Hill |
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.