Abeloff:
Clinical Oncology,
TREATMENT OF TUMOR INDUCED HYPERCALCEMIA
The serum calcium concentration can be lowered in almost all
patients with tumor-induced hypercalcemia.
A wide variety of antihypercalcemic regimens remains in common use, although
the wide therapeutic index and high success rates of the newer bisphosphonates
has resulted in their use as first-line management in the majority of cases.
Although it has been possible to target osteoclast-mediated bone resorption in
a fairly specific way, the same cannot be said for enhanced renal tubular
calcium reabsorption or gastrointestinal calcium absorption.
Selection of therapy should be geared to a knowledge of the
individual tumor type (and hence the likely mechanism underlying the hypercalcemia), and to the state of the
patient's renal function and bone marrow reserve. Any specific antineoplastic
therapy that can be used, be it surgical, radiotherapeutic, or
chemotherapeutic, will be a powerful adjuvant to antihypercalcemia therapy.
Ethical Considerations
The first decision is whether or not to treat this complication.
Unless specific antitumor therapy is available, the majority of patients who
develop hypercalcemia of
malignancy are in the last few weeks of their lives (Fig. 31 - 5) . Thus,
it can be argued that treatment is not indicated for all cases of hypercalcemia associated with malignancy.
For some, however, the use of an effective, safe treatment to ameliorate the
substantial morbidity of hypercalcemia
and to allow patients to return home, is clearly warranted.
General Considerations
The best treatment is one directed specifically and effectively at
the underlying malignant disease. Early mobilization is a laudable but often
unachievable goal in patients with advanced malignant disease. Thiazide
diuretics should be avoided, since they promote renal tubular calcium
reabsorption.
While dietary restriction of calcium seems intuitively
appropriate, gastrointestinal calcium absorption is low in most cases of hypercalcemia associated with malignant
disease. A notable exception is in patients whose tumors are equipped with a
substrate-dependent 1alpha-hydroxylase which allows continued production of
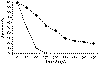 Figure
31-5 Survival curves for cancer patients with hypercalcemia. Solid circles, specific
anticancer therapy; open circle, no anticancer therapy. (Modified from Ralston SH, Gallacher SJ, Patel U, et
al: Cancer-associated hypercalcemia:
morbidity and mortality. Ann Intern Med 112:499, 1990, with permission.)
Figure
31-5 Survival curves for cancer patients with hypercalcemia. Solid circles, specific
anticancer therapy; open circle, no anticancer therapy. (Modified from Ralston SH, Gallacher SJ, Patel U, et
al: Cancer-associated hypercalcemia:
morbidity and mortality. Ann Intern Med 112:499, 1990, with permission.)
calcitriol. Patients taking supplemental
vitamin D and vitamin A (beta-carotene) should be advised of the hypercalcemic
effects of these agents.
Extracellular Fluid Volume Expansion
Most patients with hypercalcemia
of malignancy are significantly volume depleted (on the order of 5 to 10 L),
due to the combined effects of anorexia, vomiting, and nephrogenic diabetes
insipidus. In this state, the glomerular filtration rate is reduced and the
proximal convoluted tubular response is to increase sodium retention.
Concomitantly, proximal tubular resorption of calcium is also increased. The
aim of fluid replacement in these circumstances should be to induce a state of
mild fluid overload. Restoration of a normal circulating blood volume will
restore the glomerular filtration rate and increase the fractional excretion of
calcium. Further salt loading will induce a natriuresis and a concomitant
calciuresis. Care must be taken to avoid severe congestive cardiac failure in
elderly patients or patients with poor cardiac reserve. Because of the
hypoalbuminemia that frequently accompanies advanced malignant disease,
dependent edema is to be expected during volume expansion. Care must also be
taken to ensure an adequate intake of free water. In the presence of severe hypercalcemia, a resistance to the distal
tubular actions of antidiuretic hormone may predispose obtunded patients to
significant hypernatremia. After restoration of euvolemia, a maintenance
infusion of 3 L/d of 0.9 percent saline solution will induce a continued
natriuresis. Patients should be encouraged to drink freely. During such
aggressive fluid management, other electrolyte abnormalities are likely to be
uncovered or precipitated. Despite impaired renal function, both hypokalemia
and hypomagnesemia are frequent findings, and appropriate supplementation may
be required.
While the serum calcium can be expected to fall on this regimen,
restoration of normocalcemia is unlikely
(Fig. 31 - 6) . A
failure to restore normal fluid balance, however, will greatly detract from the
success of subsequent therapeutic measures.
Calciuretic Therapy
Aside from the calciuretic effects of saline overload, two other
agents are commonly employed to induce renal calcium wasting.
Furosemide
Furosemide is a diuretic agent whose main site of action is in the
thick ascending limb of the loop of Henle ( loop
diuretic), where it completely and reversibly inhibits the Na+ /K+ /2Cl- co-transporter. In the euvolemic and
volume-expanded state, the fractional excretion of calcium can be increased by
up to 30 percent by loop diuretics. However, if the patient is volume depleted,
enhanced proximal tubular sodium and calcium resorption can obviate this
response. Thus the potential exists for loop diuretics to aggravate hypercalcemia if adequate attention is not paid to volume
status. In the initial report of the effectiveness of this treatment the
regimen involved the administration of doses of furosemide in the region of 100
mg every 2 hours. [53] This aggressive therapy would require
the facilities of an intensive care unit to ensure adequate fluid monitoring.
While substantial falls in the serum calcium can be achieved, a rationale for
the use of this treatment for other than very acute situations is lacking, in
that the primary cause of the hypercalcemia
-- increased bone resorption -- is not affected. Given the risks of severe
electrolyte disturbances and the availability of potent antiresorptive
medication, the use of loop diuretics should be primarily reserved for
situations of fluid overload, rather than used as antihypercalcemic agents.
Calcitonin
The renal actions of calcitonin are complex. The calciuretic
effect appears to be due to inhibition of calcium reabsorption in the distal
tubules. This, in turn, is dependent on an adequate delivery of calcium to the
distal nephron, a situation that is compromised by the extracellular fluid
volume depletion in hypercalcemia.
However, this renal tubular effect is rapid, and in a study by Hosking and
Gilson [54] accounted for a mean fall of 0.35 ±
0.057 mmol/L in 11 patients who responded well (Fig. 31 - 7) . This
can be of great value as an adjunct to more potent antiresorptive therapies.
Antiresorptive Therapy
Given that bone resorption is increased in the majority of cases
of hypercalcemia of malignancy,
the best treatment after that designed to combat the tumor itself is one
directed at bone resorption. The osteoclasts represent the final common pathway
for bone resorption in both humoral and local osteolytic hypercalcemia. The following agents, which
inhibit osteoclast function, not surprisingly are highly effective antihypercalcemia
treatment.
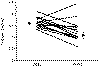 Figure
31-6 Effect of rehydration on 16 hypercalcemic patients. (Data from Hosking DJ, Cowley A, Bucknall A:
Rehydration in the treatment of severe hypercalcemia.
Q J Med 200:473, 1981.)
Figure
31-6 Effect of rehydration on 16 hypercalcemic patients. (Data from Hosking DJ, Cowley A, Bucknall A:
Rehydration in the treatment of severe hypercalcemia.
Q J Med 200:473, 1981.)
Bisphosphonates
The bisphosphonates are a class of compounds, structural analogues
to pyrophosphate (PPi ), in which the P-O-P bond is replaced by a P-C-P bond
stable to enzymatic cleavage. Figure 31 - 8 shows the
structure of some of the available bisphosphonates as compared with that of PPi .
Pharmacokinetic and pharmacodynamic studies of bisphosphonates
indicate that these compounds are absorbed poorly from the gastrointestinal
tract after oral administration. Studies in healthy adult male volunteers [55] [56] have shown that gastrointestinal
absorption is on the order of 1 to 3 percent, but varies between individuals.
Diet has a profound effect on gastrointestinal absorption, reducing the
effective availability of the drug to zero if it is taken with food. [57] Once absorbed, it appears that
approximately 50 percent of the compound is excreted unchanged in the urine,
the rest being sequestered in bone and soft tissue, [58] where its
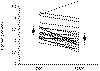 Figure
31-7 Effect of calcitonin (100 U/d) in 21 hypercalcemic patients. (Data from Hosking DJ, Gilson D: Comparison of the
renal and skeletal actions of calcitonin in the treatment of severe hypercalcemia of malignancy. Q J Med
211:359, 1984.)
Figure
31-7 Effect of calcitonin (100 U/d) in 21 hypercalcemic patients. (Data from Hosking DJ, Gilson D: Comparison of the
renal and skeletal actions of calcitonin in the treatment of severe hypercalcemia of malignancy. Q J Med
211:359, 1984.)
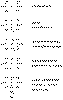 Figure
31-8 Structural formulae of commonly studied bisphosphonates in relation
to the generic biphosphonate and to pyrophosphate.
Figure
31-8 Structural formulae of commonly studied bisphosphonates in relation
to the generic biphosphonate and to pyrophosphate.
half-life (in rats) is 4 months. [59] From the experiments of Jung and
colleagues, [60] it appears that bone mineral has a very
high affinity for bisphosphonates. Although the bulk of any absorbed
bisphosphonate is rapidly relocated to bone, this is nonhomogeneous, and varies
with the state of bone activity. Indeed, this principle is made use of for
isotope bone scanning using 99m Tc-labeled bisphosphonates. Furthermore, it is likely that
the release of bisphosphonate will be enhanced in areas of rapid bone turnover,
leading to an unpredictable recirculation of the drug. [61]
The precise mechanism of action of these compounds is unclear.
Evidence for a marked physicochemical effect of bisphosphonates came from work
by Robertson and colleagues, [62] who demonstrated that compounds such as
PPi
and bisphosphonates had major effects on the ionic makeup of the hydration
layer usually surrounding hydroxyapatite crystals in suspension. This
interaction alters the calcium phosphate product both at the surface of the
crystal and in the immediate vicinity, and so inhibiting further crystal development
and dissolution. An effort has been made to relate physicochemical effects to
the structure of a great many bisphosphonates, but it became rapidly clear that
the physicochemical interactions between bisphosphonates and bone mineral
represented only a small part of the spectrum of activities of these agents,
and no correlation between structure and activity could be demonstrated. [63]
Lysosomal enzyme systems in osteoclasts have been implicated in
the process of bone resorption, [64] and it is possible that inhibition of
these enzymes, notably the acid hydrolases but also the enzymes responsible for
energy production and protein synthesis by bisphosphonates, could account in
some way for their effects. [65] [66]
In an extensive coverage of the cellular morphologic changes
induced by bisphosphonates, Plasmans and colleagues [67] investigated changes in osteocyte,
osteoblast, and osteoclast morphology induced by etidronate in a model of
heterotopic bone formation. They discovered that the normal ruffled border of
the osteoclasts appeared to shrink, and coined the term frustrated osteoclasts. Furthermore,
transient abnormal calcium storage in the mitochondria of osteoblasts was
noted, and the osteocytes appeared to be stimulated into greater activity, with
enhancement of subcellular organelles. Although in many in vivo studies, no
evidence for a failure in the development of osteoclasts had been shown, in
vitro work by Boonekamp and colleagues [68] has suggested that pamidronate, but not
etidronate or clodronate, was able to inhibit the accession of mononuclear
osteoclast precursors into a previously osteoclast-free system.
Etidronate.
Etidronate (1-hydroxy-ethylidene-1,1-bisphosphonate) was the first
bisphosphonate licensed for use in the management of metabolic bone disease.
Oral administration of this agent became well established in the treatment of
Paget's disease of bone, and clinical trials have shown it may be of use in the
management of osteoporosis. [69] [70] Like all currently available
bisphosphonates, etidronate suffers from poor and unpredictable oral
bioavailability. The hypocalcemic response to intravenous etidronate is not as
marked as with newer bisphosphonates (Fig. 31 - 9) . A
conservative estimate suggests that normocalcemia can be restored in
approximately 40 percent of patients. Evidence for a sequential beneficial
effect of oral etidronate following normalization of serum calcium exists, but
is poor (see "Long-Term Treatment," below). Etidronate differs from
other bisphosphonates studied in that its use is frequently associated with
hyperphosphatemia.
Clodronate.
The use of intravenous clodronate (dichloromethylene
bisphosphonate) for the control of the hypercalcemia
of malignancy was investigated by several groups in the United States and
Europe in the early 1980s. The appearance of three cases of acute leukemia in
664 patients led to the temporary withdrawal from use of this agent pending
analysis of the likelihood of leukemia being a true side effect. [72] Thereafter, much of the investigation of
this agent has been limited to groups in Europe and, more recently, Canada,
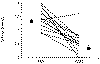 Figure
31-9 Effect of etidronate (50 to 1,000 mg/d) in 13 hypercalcemic
patients. (Data from Jung A: Comparison of
two parenteral diphosphonates in hypercalcemia of malignancy. Am J Med
72:221, 1982.)
Figure
31-9 Effect of etidronate (50 to 1,000 mg/d) in 13 hypercalcemic
patients. (Data from Jung A: Comparison of
two parenteral diphosphonates in hypercalcemia of malignancy. Am J Med
72:221, 1982.)
where clodronate has approval for use as
an antihypercalcemic agent.
Clodronate is a very effective agent in restoring normocalcemia.
Oral clodronate also appears able to induce a normocalcemic response, [73] but in the clinical situation of the
acute management of hypercalcemia,
the intravenous route is preferred.
Impairment of renal function has been reported in patients receiving
rapid infusions of intravenous clodronate in the setting of multiple myeloma. [74] It is unclear if a direct
cause-and-effect relationship holds, given the underlying renal complications
in patients with multiple myeloma.
Pamidronate.
Pamidronate disodium was the first of the aminobisphosphonates
licensed for clinical use. Like clodronate, pamidronate is a very effective
agent at restoring normocalcemia. [75] An early dose-response study from Europe
carried out by Body and colleagues in 1987 [76] showed little advantage to increasing
the dose beyond 0.25 mg/kg. More recently, however, Thiebaud and colleagues [77] showed a more impressive dose-response
effect using single intravenous infusions of pamidronate (from 30 to 90 mg) in
terms of both restoration of normocalcemia and duration of the normocalcemic
response. This finding has been confirmed in a large multicenter trial in the
United States. [6] In the early studies, pamidronate was
given in divided doses over a period of several days. It is possible, however,
to achieve the same effect with single-dose therapy. [78] Dodwell and colleagues have studied more
rapid infusion rates [79] and have shown that the drug can be
given safely and efficaciously over a 2-hour period.
Comparative studies involving
bisphosphonates.
An early study involving 30 patients with hypercalcemia and malignant disease showed
that, while volume repletion had only minor beneficial effects in terms of
lowering the serum calcium level (3.15 ± 0.12 to 3.0 ± 0.11 mmol/L),
pamidronate disodium in doses ranging from 15.75 to 300 mg (over 3 to 10 days)
lowered the serum calcium on average from 3.0 ± 0.11 to 2.19 ± 0.07. [80] Ralston and colleagues [81] compared the hypocalcemic effect of
pamidronate disodium with that of mithramycin and a combination of
corticosteroids and calcitonin. They demonstrated that pamidronate induced a
more effective fall in serum calcium than either mithramycin or the combination
of corticosteroids and calcitonin, although the time to the onset of the effect
was longer. Thurlimann and colleagues [82] performed a comparative, randomized,
crossover study to compare the hypocalcemic effects of a single intravenous
dose of 60 mg pamidronate with 20 mug/kg mithramycin. There were no primary
failures in the pamidronate-treated group (11 of 11), but 6 of 14 patients in
the mithramycin-treated group failed to achieve normocalcemia. When those
patients, along with two others from this group who had become hypercalcemic
again, were treated with pamidronate, all eight became normocalcemic.
A comparative study involving the three commonly available
bisphosphonates in Europe demonstrated that a single intravenous infusion of 30
mg pamidronate induced a more rapid and more pronounced fall in serum calcium
than 600 mg of clodronate as a single intravenous dose or 7.5 mg/kg etidronate
intravenously for 3 days. [83] A multicenter U.S. study comparing the
hypocalcemic effects of etidronate and pamidronate confirmed the superiority of
the latter bisphosphonate. [84]
Effect of tumor type on response
to bisphosphonates.
Although the primary mechanism in the generation and maintenance
of hypercalcemia in malignant
disease is enhanced bone resorption, tumor types differ significantly in the
way they bring this about. Morton and colleagues [75] could detect no statistically
significant difference between hypercalcemic patients with squamous carcinoma
of the bronchus ( n = 12),
carcinoma of the breast ( n = 6),
or multiple myeloma ( n = 5) in
their response to 60 mg pamidronate. More recently, it has been suggested that
a low renal phosphate threshold (indicative of the effects of PTHrP) may be
used to indicate the likelihood of a poor response to treatment, [85] and Dodwell and colleagues [86] have demonstrated a statistically
significant relationship between the circulating PTHrP concentration and the
time to normalization of hypercalcemia.
Duration of response to
bisphosphonate therapy.
The duration of response to bisphosphonates is difficult to
determine and varies considerably between individuals. Elucidation of the
duration of response is also compounded by the high mortality in this group of
patients due to their tumor, and by the introduction of specific and effective
antineoplastic therapy for patients with cancer of the breast and multiple
myeloma. In the patients treated by Morton and colleagues, [75] only 11 of 30 patients (37 percent)
survived longer than 1 month following the onset of their hypercalcemia. In this study, the median time
to recurrence of hypercalcemia was
approximately 3 weeks. The same median duration of normocalcemia was also found
by Harinck and
Bijvoet [87] ; however, a longer one of 35 days was
reported by Thiebaud and colleagues. [77]
Unfortunately, it is not possible to predict the length of time
that any specific patient will remain normocalcemic.
Side effects of bisphosphonates.
In general, bisphosphonate therapy is well tolerated. Low-grade
pyrexia is noted in 10 to 15 percent of patients. The use of rapid intravenous
infusions of clodronate and etidronate has been associated with deterioration
in renal function in patients with previously diminished renal reserve.
Hyperphosphatemia is noted with etidronate therapy, but hypophosphatemia is
seen with clodronate and pamidronate treatment. The mechanisms of phosphate imbalance
are unclear, although etidronate appears to have a specific effect on renal
phosphate handling. [88] Prolonged use of etidronate has been
associated with a fracturing osteomalacia in patients with Paget's disease of
bone, but this is of little relevance in hypercalcemic individuals. Oral
bisphosphonates are associated with gastrointestinal intolerance, and are
probably best avoided in the acute situation.
New bisphosphonates.
Alendronate disodium is a potent aminobisphosphonate that is being
used in an oral form in the management of osteoporosis. A dose-response study
demonstrated that alendronate 10 mg intravenously over 2 hours restored normal
calcium levels in 90 percent of patients. [89] No long-term studies with oral
alendronate in hypercalcemic patients are available.
Ibandronate is a bisphosphonate that is approximately 50 times
more potent than pamidronate in animal models of bone resorption. A
dose-response study of this agent showed that an intravenous dose of 6 mg was
highly effective at restoring normal calcium levels. [90] The highly potent cyclic imidazole
bisphosphonate zoledronate is also highly effective at restoring normocalcemia
at doses of 0.02 to 0.04 mg/kg.
Despite increases in relative potency, it is unlikely that the
newer bisphosphonates will achieve better response rates than pamidronate.
Advantages of newer agents may include more rapid infusion times and alternate
routes of drug delivery.
Plicamycin (Mithramycin)
This bacteriostatic antibiotic agent was used in the late 1960s
for the treatment of germ cell tumors. [91] A side effect of this agent in
normocalcemic individuals was hypocalcemia. The mechanism of the hypocalcemia
is unclear, although in vitro studies suggest that a direct toxic effect on
osteoclasts is responsible. [92] Despite a long list of side effects, including
marrow, hepatic, and renal toxicity, plicamycin remains a widely used agent for
the management of hypercalcemia.
Plicamycin is effective at restoring normocalcemia in approximately 80 percent
of patients, and many consider the toxic effects to be overstated, since the
antihypercalcemic effect is seen at doses 10 times lower than those used in the
original antineoplastic regimens. A standard infusion of 25 mug/kg over 4 to 6
hours is most often used. Longer durations of infusion may reduce the nausea
caused by this agent, but will add to the risk of extravasation and local
irritation. The hypocalcemic effect is seen within the first 24 hours, but the
duration of response is unpredictable.
One serious reported drawback with plicamycin is the development
of severe, rapid, rebound hypercalcemia
that occurs in an unpredictable fashion. [93] Furthermore, most of the toxic effects
of plicamycin are cumulative. Thus, its use in the long-term management of hypercalcemia is limited.
While the safer (and more effective) aminobisphosphonates have
replaced plicamycin as first-line antiresorptive therapy, this agent may be of
use in the infrequent individual whose hypercalcemia
proves resistant.
Calcitonin
Since calcitonin has both calciuretic and antiresorptive actions,
it would appear that this would be the ideal antihypercalcemic agent. The
antiresorptive effects of calcitonin are related to a direct osteoclast
toxicity, and, possibly to inhibition of new osteoclast recruitment. When used
as a single agent, the hypocalcemic effect of calcitonin is modest at best, and
resistance to the effects of calcitonin develops rapidly.
Nonetheless, a major role for calcitonin in combination with
powerful antiresorptive agents is emerging. Fatemi and colleagues [94] reported an enhanced and more rapid
hypocalcemic effect with the combination of etidronate and calcitonin, as had
Ralston and colleagues [91] with pamidronate and calcitonin.
While originally recommended for subcutaneous use, newer routes of
delivery, including suppositories and nasal sprays, have been developed with
varying levels of efficacy.
In cases of life-threatening hypercalcemia,
or where neurologic symptoms are a major feature, we recommend the use of 8 MRC
(Medical Research Council) units/kg given intramuscularly every 6 hours for 1
or 2 days in association with an intravenous bisphosphonate. This regimen has
the advantage of combining the rapid calciuretic effect of calcitonin with the
powerful and prolonged antiresorptive effect of the bisphosphonate. [95]
Gallium Nitrate
Hypocalcemia was noted as a side effect of therapy in patients
receiving gallium nitrate for the management of lymphoma. [96] Thereafter, its effectiveness was
confirmed by Warrell and colleagues [97] in open-labeled studies and in a
randomized, double-blind comparative study with calcitonin. [98] The mechanism of action of gallium is
unknown, although it is clear that urinary calcium excretion is reduced. By
implication, bone resorption is reduced, although no histologic changes were
noted in explants of fetal long bones exposed to this agent.
Gallium nitrate requires intravenous administration. The best
investigated regimens involve sequential 5-day
|
MANAGEMENT OF HYPERCALCEMIA OF MALIGNANCY The most effective way to control the hypercalcemia of malignant disease is by
therapy aimed at eradicating or reducing the tumor burden. Chemotherapy,
radiation therapy, and surgical therapy all have a role to play. In the
absence of effective antitumor therapy, the patient's general condition and
immediate prognosis should be used to guide the decision to embark on
aggressive antihypercalcemic therapy or active palliation, or both. The
introduction of agents with high efficacy and few side effects has broadened
the oncologist's options. Our practice is to discuss treatment options with the
patients and their families, emphasizing that the drugs used to control the hypercalcemia will not influence the
progression of the underlying cancer, but will help the symptoms of the hypercalcemia. Volume expansion with 0.9
percent saline is begun immediately. The rate is determined by the state of
hydration of the individual patient as assessed by the clinician. An infusion
of intravenous pamidronate is begun at the same time as saline volume
expansion. The dose of pamidronate chosen depends on the corrected serum
calcium level. For a calcium concentration of 3.0 mmol/L or greater, a dose
of 90 mg pamidronate over 24 hours is used. For a calcium concentration less
than 3.0 mmol/L, a dose of 60 mg pamidronate infused over 8 hours is used. In
the presence of severe hypercalcemia
and neurologic symptomatology, calcitonin 8 MRC units/kg intramuscularly
every 6 hours is used in conjunction with the pamidronate. Biochemical response is rapid. The serum calcium can be
expected to fall after 24 hours. Most patients reach a nadir calcium value in
5 to 7 days. We maintain a natriuresis by continuing the saline infusion
until normocalcemia is reached. Volume overload, as evidenced by an elevation
of the jugular venous pressure, the development of a fourth heart sound,
pulmonary congestion, or peripheral edema, is treated using furosemide, which
has the added benefit of inducing a calciuresis. Care is taken to avoid
volume depletion when using the diuretic. Close attention is paid to renal function
and electrolyte balance, since hypokalemia, hypomagnesemia, and
hypophosphatemia are common sequelae of this treatment approach. In cases
resistant to the initial dose of pamidronate, re-treatment can be given at a
dose 30 mg higher than the previously attempted dose to a maximum of 180 mg
over 24 hours. Failure to respond to bisphophonate is a poor prognostic
feature, but alternative antiresorptive therapy can be attempted (gallium
nitrate, plicamycin). In the absence of effective antitumor therapy, the hypercalcemia will almost certainly recur
if the patient survives long enough. The duration of normocalcemia is
variable, and further antihypercalcemic therapy must be individualized.
Patients are advised to maintain a high fluid intake (3 L/d). Corrected
calcium concentration is determined weekly. We re-treat patients when the
corrected serum calcium exceeds 2.7 mmol/L, and at regular intervals
thereafter. Re-treatment is done on an outpatient basis when possible. The
dose of pamidronate is based on the last dose that reversed the hypercalcemia. Often the malignant process is at such an advanced stage
that death occurs within a few weeks of the development of hypercalcemia. Because of this we involve
palliative care early in the management of hypercalcemic patients. |
infusions of 200 mg/m2 /d. At this dose, the drug is relatively free of side
effects, although caution is required if other nephrotoxic agents (e.g.,
aminoglycosides) are being used.
Prostaglandin Synthesis Inhibitors
As discussed above, prostaglandins (notably prostaglandin E2 ) have potent bone resorbing effects in
relationship to certain tumor types. Thus it was hoped that a significant
subset of patients might be found who would respond to prostaglandin synthesis inhibitors
such as indomethacin. Although well-characterized case reports have shown a
good response to these agents, in general they are ineffective for the
treatment of tumor-induced hypercalcemia.
[99] Seyberth and colleagues [100] attempted to characterize those patients
who might be responsive in terms of their biochemical parameters. As one would
intuitively expect, those patients with a high urinary excretion of
prostaglandin E2 metabolites showed the best response. In general, however,
no reliance can be placed on prostaglandin synthesis inhibitors in this
clinical setting.
Other Therapies
Corticosteroids
Glucocorticoids are commonly employed in the management of
tumor-induced hypercalcemia
despite significant evidence that their usefulness is limited. The mechanism of
any hypocalcemic effect produced by these agents is unclear. In patients with
multiple myeloma and lymphoid malignancies, glucocorticosteroids form part of
the antineoplastic regimen (e.g., melphalan and prednisone, VAD,
vincristine, doxorubicin, and dexamethasone; MOPP, mechlorethamine,
vincristine, procarbazine, and prednisone; CHOP, cyclophosphamide, doxorubicin,
vincristine, and prednisone,) because of their known
cytotoxic effect on lymphoid tissue. Furthermore, glucocorticosteroids block
the absorption of calcium from the gut, thus they could be expected to be
useful in patients with vitamin D - mediated hypercalcemia
where gastrointestinal absorption of calcium is enhanced.
In patients with solid tumors, corticosteroids are not usually
effective and have no role in the management of hypercalcemia. [101]
Phosphate
The use of intravenous phosphate to complex calcium and induce
precipitation in the extracellular fluid and soft tissue can no longer the
supported. [102] Oral phosphate, however, is less toxic,
and may be tried for the long-term management of hypercalcemia. The dose-limiting side effect of the oral agent
is diarrhea. Oral phosphate, while acting primarily as a gastrointestinal
calcium chelator, may also have some inhibitory effects on osteoclast function.
Dialysis
Hemodialysis using a dialysate bath free of calcium can be used in
the emergency treatment of hypercalcemia.
However, the authors (a nephrologist and an oncologist) have never been called
upon to use this therapy in over 10 years of clinical practice.
Experimental Agents
WR-2721
The radioprotectant organic thiophosphate S-2-(3-aminopropylaminoethyl)
phosphorothioic acid, also known as WR-2721, is an agent with unique effects on
calcium metabolism. This agent inhibits parathyroid hormone secretion by an
unknown mechanism [103] but, more importantly from the point of
view of the management of the hypercalcemia
of malignancy, it also appears to inhibit PTH-independent calcium reabsorption.
[104] Although this agent is relatively
nontoxic, it is not widely available for the management of hypercalcemia.
Cis-platinum
A clinical study by Lad and colleagues [105] involving 23 episodes of hypercalcemia in 13 patients showed a
complete response rate of 69 percent. Patients were selected for whom it was
considered unlikely that the cis-platinum
would have a specific antitumor effect. The duration of the hypocalcemic effect
was on the order of 1 month. The authors comment that at the given dose (100
mg/m2
), this therapy was nontoxic.
Carbonic Anhydrase Inhibitors
The generation of an acid environment for the activation of acid
hydrolases is crucial to osteoclastic function. Protons for transport into the
extracellular lysosome at the site of bone resorption are produced by the
enzyme carbonic anhydrase II, [106] which catalyzes the hydration of carbon
dioxide to carbonic acid. Acetazolamide is a carbonic anhydrase inhibitor that
has been widely used for the treatment of glaucoma, and as a diuretic agent.
Brown and colleagues [107] demonstrated that acetazolamide caused a
significant fall in calcium level in hypercalcemic rats bearing an H500
Leydig's cell tumor. Carbonic anhydrase inhibitors also produce a metabolic
acidosis (because of renal bicarbonate wasting), and the investigators note
that this acidosis must be prevented to allow the hypocalcemic effect.
No study of the efficacy of carbonic anhydrase inhibitors in human
hypercalcemia has been published
to date.
Long-Term Treatment
In patients for whom no antitumor therapy is available, long-term
survival is unusual. By implication, there are few good long-term studies on
the management of hypercalcemia,
and most results are anecdotal. Individualization of therapy is the rule.
Patients should be advised to drink an adequate volume of fluid (2 to 3 L/
|
TABLE 31-5 -- OPTIONS FOR LONG-TERM MANAGEMENT
OF HYPERCALCEMIA OF MALIGNANCY |
||
|
AGENT |
DOSE |
FREQUENCY * |
|
60-90 mg over 2-4 h |
Every 2-3 weeks |
|
|
200-1,200 mg |
Daily |
|
|
Oral clodronate § |
3,200 mg |
Daily |
|
20 mg/kg |
Daily |
|
|
Oral phosphate |
2-3 g |
Daily |
|
Corticosteroids |
Variable |
Daily |
|
Multiple myeloma |
|
|
|
Carcinoma of the breast |
|
|
|
NSAIDs |
Variable |
Daily |
|
Abbreviation: NSAIDs, nonsteroidal
anti-inflammatory drugs. |
||
*Suggested frequencies and doses may be altered to suit individual
patients.
![]() Data
from Dodwell et al. [79]
Data
from Dodwell et al. [79]
![]() Data
from Thiebaud et al. [109]
Data
from Thiebaud et al. [109]
§ Data
from chapuy et al. [73]
![]() Data
from Ringenberg and Ritch [110]
and Hasling et al. [111]
Data
from Ringenberg and Ritch [110]
and Hasling et al. [111]
d) and to maintain their mobility for as
long as possible. They should be reminded of the symptoms of hypercalcemia and urged to present early
should those symptoms arise. Table 31 - 5 shows
suggested maintenance treatments for the hypercalcemia
of malignancy.
The importance of palliative care cannot be overemphasized in the
management of these unfortunate individuals.
A logical therapeutic regimen for the acute management of
tumor-induced hypercalcemia is
shown in the preferred treatment box. This regimen represents one approach to
this problem. Other equally valid regimens are possible, and individualization
of regimens is mandatory for long-term therapy.
REFERENCES
1.
Zondek H, Petow H, Seibert W: Die Bedeutung der Calciumbestimmung
im Blute fur die Diagnose der Niereninsuffizienz. Z Klin Med 99:129, 1924
2.
Gutman AB, Tyson LT, Gutman BE: Serum calcium, inorganic
phosphorus and phosphatase activity in hyperparthyoidism, Paget's disease,
multiple myeloma and neoplastic disease of bone. Arch Intern Med 57:379, 1936
3.
Mundy GR, Martin TJ: The hypercalcemia
of malignancy: pathogenesis and management. Metabolism 31:1247, 1982
4.
Fisken RA, Heath DA, Bold AM: Hypercalcaemia -- a hospital survey.
Q J Med 196:405, 1980
5.
Coleman RE, Rubens RD: The clinical course of bone metastases from
breast cancer. Br J Cancer 55:61, 1987
6.
Nussbaum SR, Mallette L, Gagel R, et al: Pamidronate (APD)
treatment of hypercalcaemia associated with malignancy -- preliminary report of
a multicenter double-blind trial. In Rubens RD (ed): The Management of Bone
Metastases and Hypercalcaemia by Osteoclast Inhibition. Bern, Hogrefe &
Huber, 1990, p 44
7.
Mundy GR: Calcium Homeostasis: Hypercalcemia
and Hypocalcemia. London, Martin Dunitz, 1990, p 2
8.
Parfitt AM: Equilibrium and disequilibrium hypercalcaemia: new
light on an old concept. Metabolic Bone Dis Rel Res 1:279, 1979
9.
Payne RB, Little AJ, Williams RB, Milner JR: Interpretation of
serum calcium in patients with abnormal serum proteins. Br Med J 4:643, 1973
10.
Morton AR, Hercz G: Hypercalcemia
in dialysis patients: comparison of diagnostic methods. Dialysis Transplant
20:661, 1991
11.
Potts JT Jr, Kronenberg HM, Rosenblatt M: Parathyroid hormone:
chemistry, biosynthesis and mode of action. Adv Protein Chem 35:323, 1982
12.
Tashjian AH Jr, Wright DR, Ivey JL, Pont A: Calcitonin binding
sites in bone: relationship to biological response and "escape."
Recent Prog Horm Res 34:285, 1978
13.
Stewart AF, Horst R, Deftos LJ, et al: Biochemical evaluation of
patients with cancer-associated hypercalcemia.
N Engl J Med 303:1377, 1980
14.
Albright F: Case records of the Massachusetts General Hospital, No
27461, N Engl J Med 225:789, 1941
15.
Lafferty FW: Pseudohyperparathyroidism. Medicine 45:247, 1966
16.
Simpson EL, Mundy GR, D'Souza SM, et al: Absence of parathyroid hormone
messenger RNA in nonparathyroid tumors associated with hypercalcemia. N Engl J Med 309:325, 1983
17.
Burtis WJ, Wu T, Bunch C, et al: Identification of a novel
17,000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein
from a tumor associated with humoral hypercalcemia
of malignancy. J Biol Chem 62:7151, 1987
18.
Moseley JM, Kubota M, Diefenbach-Jagger H, et al: Parathyroid
hormone-related protein purified from a human lung cancer cell line. Med Sci
84:5048, 1987
19.
Strewler GJ, Stern PH, Jacobs JW, et al: Parathyroid hormone like
protein from human renal carcinoma cells. J Clin Invest 80:1803, 1987
20.
Kukreja SC, Shevrin DH, Wimbiscus SA, et al: Antibodies to
parathroid hormone-related protein lower serum calcium in athymic mouse models
of malignancy-associated hypercalcemia
due to human tumors. J Clin Invest 82:1798, 1988
21.
Budayr AA, Nissenson RA, Klein RF, et al: Increased serum levels
of a parathyroid hormone-like protein in malignancy-associated hypercalcemia. Ann Intern Med 111:807, 1989
22.
Ratcliffe WA, Hutchesson ACJ, Bundred NJ, Ratcliffe JG: Role of
assays for parathyroid-hormone-related protein in investigation of
hypercalcaemia. Lancet 339:164, 1992
23.
Goltzman D, Henderson JE: Parathyroid hormone-related peptide and hypercalcemia of malignancy. In Arnold A
(ed): Endocrine Neoplasms. Kluwer Academic Publishers, 1997
24.
Mundy GR, Raisz LG, Cooper RA, et al: Evidence for the secretion
of an osteoclast stimulating factor in myeloma. N Engl J Med 291:(20):1041,
1974
25.
Dewhirst FE, Stashenko PP, Mole JE, Tsurumachi T: Purification and
partial sequence of human osteoclast-activating factor: identity with
interleukin 1beta. J Immunol 135:2562, 1985
26.
Garrett R, Durie BGM, Nedwin GE, et al: Production of lymphotoxin,
a bone-resorbing cytokine, by cultured human myeloma cells. N Engl J Med
317:526, 1987
27.
Klein B, Bataille R: Cytokine network in human multiple myeloma.
Hematol Oncol Clin North Am 6:273, 1992
28.
Al-Humidan A, Ralston SH, Hughes DE, et al: Interleukin 6 does not
stimulate bone resorption in neonatal mouse calvariae. J Bone Miner Res 6:3,
1991
29.
Ishimi Y, Miyaura C, Jin CH, et al: IL-6 is produced by
osteoblasts and induces bone resorption. J Immunol 145:3297, 1990
30.
Galasko CSB: Mechanisms of bone destruction in the development of
skeletal metastases. Nature 263:507, 1976
31.
Perroteau I, Salomon D, DeBortoli M, et al: Immunological
detection and quantitation of alpha transforming growth factors in human breast
carcinoma cells. Breast Cancer Res Treat 7:201, 1986
32.
Tashjian A Jr, Voelkel EF, Lloyd W, et al: Actions of growth
factors on plasma calcium. J Clin Invest 78:1405, 1986
33.
Klein DC, Raisz LG: Prostaglandins: stimulation of bone resorption
in tissue culture. Endocrinology 86:1436, 1970
34.
Galasko CSB, Bennett A: Relationship of bone destruction in
skeletal metastases to osteoclast activation and prostaglandins. Nature
263:508, 1976
35.
Powles TJ, Clark A, Easty DM, et al: The inhibition by aspirin and
indomethacin of osteolytic tumour deposits and hypercalcaemia in rats with
Walker tumour, and its possible application to human breast cancer. Br J Cancer
28:316, 1973
36.
Percival RC, Yates AJP, Gray RES, et al: Mechanisms of malignant hypercalcemia in carcinoma of the breast.
Br Med J 291:776, 1985
37.
Bundred NJ, Ratcliffe WA, Walker RA, et al: Parathyroid hormone
related protein and hypercalcaemia in breast cancer. Br Med J 303:1506, 1991
38.
Budyar AA, Halloran BP, King JC, et al: High levels of
parathyroid-like protein in milk. Proc Natl Acad Sci USA 86:7182, 1989
39.
Barbour GL, Coburn JW, Slatopolsky E, et al: Hypercalcemia in an anephric patient with
sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N
Engl J Med 305:440, 1981
40.
Davies M, Mawer EB, Hayes ME, Lumb GA: Abnormal vitamin D
metabolism in Hodgkin's lymphoma. Lancet i:1186, 1985
41.
Canellos GP: Hypercalcemia
in malignant lymphoma and leukemia. Ann N Y Acad Sci 230:240, 1974
42.
Breslau NA, McGuire JL, Zerwekh JE, et al: Hypercalcemia associated with increased
serum calcitriol levels in three patients with lymphoma. Ann Intern Med 100:1,
1984
43.
Rosenthal N, Insogna KL, Godsall WJ, et al: Elevations in
circulating 1,25-dihydroxyvitamin D in three patients with lymphoma-associated hypercalcemia. J Clin Endocrinol Metab
60:29, 1985
44.
Adams JS, Fernandez M, Gacad MA, et al: Vitamin D
metabolite-mediated hypercalcemia
and hypercalciuria patients with AIDS- and non-AIDS-associated lymphoma. Blood
73: 235, 1989
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.