![]()
Patients at High Risk of
Death after Lung-Volume–Reduction Surgery
National Emphysema Treatment Trial Research Group
Notice: Because of its clinical implications, this article is being
published on August 14, 2001. It will appear in the October 11 issue of the New England Journal of Medicine.
ABSTRACT
Background Lung-volume–reduction
surgery is a proposed treatment for emphysema, but optimal selection criteria
have not been defined. The National Emphysema Treatment Trial is a randomized,
multicenter clinical trial comparing lung-volume–reduction surgery with medical
treatment.
Methods After
evaluation and pulmonary rehabilitation, we randomly assigned patients to
undergo lung-volume–reduction surgery or receive medical treatment. Outcomes
were monitored by an independent data and safety monitoring board.
Results A total of
1033 patients had been randomized by June 2001. For 69 patients who had a
forced expiratory volume in one second (FEV1) that was no more than
20 percent of their predicted value and either a homogeneous distribution of
emphysema on computed tomography or a carbon monoxide diffusing capacity that
was no more than 20 percent of their predicted value, the 30-day mortality rate
after surgery was 16 percent (95 percent confidence interval, 8.2 to 26.7
percent), as compared with a rate of 0 percent among 70 medically treated
patients (P<0.001). Among these high-risk patients, the overall mortality
rate was higher in surgical patients than medical patients (0.43 deaths per
person-year vs. 0.11 deaths per person-year; relative risk, 3.9; 95 percent
confidence interval, 1.9 to 9.0). As compared with medically treated patients,
survivors of surgery had small improvements at six months in the maximal
workload (P=0.06), the distance walked in six minutes (P=0.03), and FEV1
(P<0.001), but a similar health-related quality of life. The results of the
analysis of functional outcomes for all patients, which accounted for deaths
and missing data, did not favor either treatment.
Conclusions Caution is
warranted in the use of lung-volume–reduction surgery in patients with
emphysema who have a low FEV1 and either homogeneous emphysema or a
very low carbon monoxide diffusing capacity. These patients are at high risk
for death after surgery and also are unlikely to benefit from the surgery.
Lung-volume–reduction surgery is a potentially valuable treatment
for patients with advanced emphysema.1 2 3 4 5 6 7 8
During the operation, 20 to 35 percent of the emphysematous lung is resected by
means of either a median sternotomy or video-assisted thoracoscopy. Generally,
lung function, exercise capacity, and the quality of life improve after
surgery, but the results vary.9
The surgical mortality rate ranges from 4 to 15 percent,3
and one-year mortality rates are as high as 17 percent,10
although follow-up has often been incomplete.11
A review of Medicare claims showed that the six-month mortality rate was 16.9
percent.12
Uncertainty about the risk of lung-volume–reduction surgery, the magnitude and
duration of benefit, and optimal selection criteria led the National Heart,
Lung, and Blood Institute and the Center for Medicare and Medicaid Services
(formerly the Health Care Financing Administration) to sponsor a multicenter,
randomized clinical trial, the National Emphysema Treatment Trial.13
The main goal of the trial is to compare survival rates and
exercise capacity two years after lung-volume–reduction surgery with the
results obtained after medical treatment. An important goal of the trial is to
identify selection criteria for lung-volume–reduction surgery. The inclusion
criteria for the trial are broad enough to allow the evaluation of subgroups of
patients who have traditionally been considered candidates for surgery, but who
were present in only small numbers in previous studies.10
Every three months a data and safety monitoring board reviews recent medical
literature, the quality of the data, adverse events, and outcome data from the
trial. The board is charged with periodically reviewing subgroups of patients
who may benefit from or be harmed by the procedure; as a result of such review,
a set of clinical characteristics that defines a group of patients with a high
mortality rate and little benefit after lung-volume–reduction surgery has been
identified and is described in this article. The National Emphysema Treatment
Trial has now modified the protocol to exclude these patients. Patients who do
not meet these exclusion criteria continue to be enrolled in the trial, and
their results will be reported when the trial is completed.
Methods
The design and methods of the National Emphysema Treatment Trial
have been described previously13
and are summarized below.
Screening and Base-Line Assessments
The inclusion criteria were as follows: a forced expiratory volume
in one second (FEV1) that was no more than 45 percent of the
predicted value14
but that was at least 15 percent of the predicted value among patients who were
70 years of age or older, a total lung capacity that was at least 100 percent
of the predicted value,15
a residual volume that was at least 150 percent of the predicted value,15
a partial pressure of arterial carbon dioxide of 60 mm Hg or less (55 mm Hg in
Denver) while patients were at rest and breathing room air, a partial pressure
of arterial oxygen of at least 45 mm Hg (30 mm Hg in Denver) while patients
were at rest and breathing room air, an ability to walk farther than 140 m (459
ft) in six minutes, an ability to complete three minutes of pedaling on a
bicycle ergometer without a load, and abstinence from smoking for six months
before randomization. Patients had to complete a measurement of carbon monoxide
diffusing capacity but were not excluded on the basis of the value.16
Lung function was tested according to the guidelines of the American Thoracic
Society.17 18 19
Patients were excluded if they had other medical conditions that made them
unsuitable for surgery or that might interfere with follow-up. All patients
provided written informed consent, and the study was approved by the
institutional review board at each center.
The severity and distribution of emphysema were determined from
high-resolution computed tomographic (CT) scans of the chest obtained during
full inspiration. Each lung was divided into three apical-to-basal zones, and
each zone was scored visually by a radiologist who had been trained in the
study protocol. The extent of emphysema was graded from 0 to 4, with a grade of
0 indicating no emphysema and a grade of 4 indicating the presence of emphysema
in more than 75 percent of the lung zone.20 21 22
Heterogeneous emphysema was defined as a difference in scores of at least two
among the three zones in one lung; otherwise, the distribution of emphysema was
classified as homogeneous.
The initial evaluation included six-minute walk tests,23 24
lung-function tests, bicycle ergometry to determine maximal exercise capacity,
the 77-item Quality of Well-Being questionnaire (scores can range from 0 to 1,
and higher scores indicate a better quality of life),25
echocardiography, radionuclide pharmacologic (dobutamine) stress testing,
measurement of arterial blood gases, and lung-perfusion scanning. Patients who
met the enrollment criteria had to complete 6 to 10 weeks of pulmonary
rehabilitation, after which the participating center's pulmonologist and
surgeon, in consultation with an anesthesiologist and, if necessary, a
cardiologist, had to determine whether the patient was a suitable candidate for
lung-volume–reduction surgery. Exercise testing, lung-function testing, the
Quality of Well-Being questionnaire, and six-minute walk testing were then
repeated. Patients who were randomly assigned to medical therapy continued
pulmonary rehabilitation and medical treatment. Patients who were randomly
assigned to undergo lung-volume–reduction surgery underwent bilateral surgery
by means of either a median sternotomy or video-assisted thoracoscopy; the goal
was to resect 20 to 35 percent of each lung. After surgery, patients continued
rehabilitation and medical treatment. Pulmonary-function testing, exercise
testing, the Quality of Well-Being questionnaire, and the six-minute walk test
were repeated six months after randomization.
Statistical Analysis
We ascertained vital status as of June 2001. In the calculations
of 30-day surgical mortality rates we included only patients who actually
underwent lung-volume–reduction surgery within the trial. Other analyses were
conducted according to the intention-to-treat principle and included patients
in their assigned group regardless of the treatment received. We used
contingency tables to estimate the relative risk of death between treatment
groups, and we used the Poisson distribution to calculate 95 percent confidence
intervals.26
Kaplan–Meier survival curves from the date of randomization were compared with
use of the log-rank test.27
We compared functional outcomes in survivors of surgery and medically treated
patients six months after enrollment using two-sample t-tests of the mean
change from base line. To account for deaths and missing information, we used
the following scoring system to define the change in functional outcome at six
months: patients who had died were given a score of 0, patients who did not
complete the evaluation were given a score of 1, and other patients were given
a score ranging from 2 to 10, depending on the size of the change. For bicycle
ergometry, patients who could not pedal for three minutes without a load were
classified as unable to complete testing. Patients who had died were given a score
of 0 on the Quality of Well-Being questionnaire. Patients who did not complete
the questionnaire were assigned a value equal to one half the lowest score. We
compared the distributions of scores between groups using the Wilcoxon rank-sum
exact test.28
All P values were two-sided.
Interim Monitoring
At the outset of the study, the investigators provided the data
and safety monitoring board with stopping guidelines that were to be used to
identify subgroups that benefited from lung-volume–reduction surgery as well as
subgroups whose risk was increased by the procedure. Both the investigators and
the data and safety monitoring board considered a 30-day surgical mortality
greater than 8 percent to be unacceptable; a stopping guideline was therefore
instituted to terminate randomization if the lower 95 percent confidence limit
for 30-day mortality exceeded 8 percent.
The investigators requested that the data and safety monitoring
board pay special attention to a subgroup of patients who were thought likely
to have substantial benefit from lung-volume–reduction surgery, with the
understanding that this group might have enrollment terminated early if such
benefit were found. The criteria for the group thought likely to benefit were
an age of 70 years or less, a postbronchodilator FEV1 of 15 to 35
percent of the predicted value, a partial pressure of arterial carbon dioxide
of 50 mm Hg or less (45 mm Hg in Denver), a residual volume greater than 200
percent of the predicted value, a low radionuclide perfusion ratio (0.2 or
less), a heterogeneous pattern of emphysema on CT scanning, and evidence of
hyperinflation on chest radiography.
The data and safety monitoring board examined these seven
candidate variables and five other variables (the carbon monoxide diffusing
capacity, maximal work capacity, quality of life, race or ethnic group, and
sex) added by the investigators and approved by the data and safety monitoring
board to identify subgroups of patients who might not benefit or might be at
risk from lung-volume–reduction surgery. Exploratory analyses were conducted
for each of these variables. Continuous measures were analyzed both on a
continuous scale and in binary categories, dichotomized at the approximate
quartile for the worst prognosis.
The data and safety monitoring board reviewed subgroups of
patients derived with these candidate variables every three months for evidence
of increased risk or benefit from lung-volume–reduction surgery as compared
with medical management. The statistical significance of the subgroup
differences for each variable was determined from a test for interaction of the
variable with treatment group, with a proportional-hazards regression model for
overall mortality.
Identification of a High-Risk Group
In April 2001, these analyses suggested that a low FEV1,
a homogeneous pattern of emphysema, and a high perfusion ratio predicted an
increased risk of overall mortality. In addition, a low FEV1 and a
low carbon monoxide diffusing capacity were associated with increased 30-day
mortality. Additional analyses of patients with a low FEV1 were then
requested by the data and safety monitoring board to determine whether
combination with the three other factors could define a subgroup of patients
who exceeded the stopping guideline for 30-day mortality. The data and safety
monitoring board, recognizing that any particular cutoff value for a continuous
variable is inherently arbitrary, also requested sensitivity analyses varying
the cutoff values for FEV1 and carbon monoxide diffusing capacity.
In May 2001, the data and safety monitoring board found that the subgroup
defined by a combination of low FEV1 and either homogeneous
emphysema or low carbon monoxide diffusing capacity satisfied the stopping
guidelines. Therefore, the data and safety monitoring board recommended stopping
enrollment of these patients. The board also found that the perfusion ratio did
not add prognostic value after the other risk factors had been accounted for.
It further concluded that the selected thresholds for FEV1 and
carbon monoxide diffusing capacity were the best, given the available data.
Because several risk factors with many potential cutoff points
were examined several times, the investigators and the data and safety
monitoring board considered whether discovery of the high-risk subgroups might
represent a type I error. We concluded, however, that the present findings are
unlikely to represent a type I error. The risk factors were identified
prospectively on the basis of experience and biologic information outside the
trial and were examined with respect to stringent prespecified stopping
criteria. In addition, longitudinal views of the data suggested consistency and
increasing statistical significance over time before the actual decision point.
Results
Between January 1998 and June 2001, 1033 patients underwent
randomization at 17 clinical centers. One hundred forty of the patients (13.6
percent) were in the group at high risk for death after lung-volume–reduction
surgery (70 in the group assigned to surgery and 70 in the group assigned to medical
therapy). The high-risk group had a very low FEV1 and either a very
low carbon monoxide diffusing capacity or homogeneous emphysema. All 140
patients had an FEV1 that was no more than 20 percent of their
predicted value. Ninety-four also had evidence of homogeneous emphysema on CT
scanning, and 87 also had a carbon monoxide diffusing capacity that was no more
than 20 percent of their predicted value. Forty-one patients met all three
criteria. The base-line characteristics of these patients were similar in the
two treatment groups (Table
1).
Treatment
Sixty-nine of the 70 patients assigned to undergo
lung-volume–reduction surgery underwent the procedure and 1 declined the
procedure; this patient was alive four months after randomization. The median
time from randomization to surgery was 10 days (range, 3 to 84). Forty-seven
patients had a median sternotomy, and 22 had video-assisted thoracoscopy. Four
of the 70 patients assigned to receive medical treatment underwent surgery
outside the trial. Two of these patients died: one died 22 months after
randomization and 1 year after surgery; and the other died 6 months after
randomization and 21 days after surgery. The other two patients were alive 13
months after surgery.
Mortality and Morbidity
There were no deaths in the medical-therapy group during the first
30 days after randomization. In contrast, the 30-day mortality rate after
surgery was 16 percent (95 percent confidence interval, 8.2 to 26.7 percent;
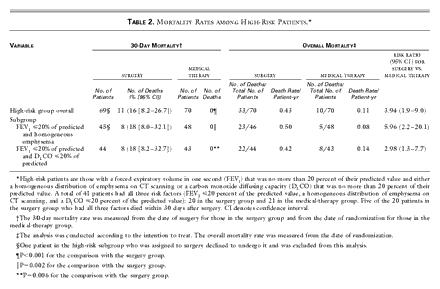
P<0.001 for the comparison with the medical group) (Table
2). Patients with all three high-risk characteristics had a 30-day mortality
rate of 25 percent (95 percent confidence interval, 8.7 to 49.1 percent) after
surgery. The 30-day mortality rate after surgery was similar among patients who
had undergone video-assisted thoracoscopy and those who had had a median
sternotomy (P>0.99).
The overall mortality rate was 0.43 deaths per person-year among
patients assigned to undergo surgery, as compared with 0.11 deaths per
person-year among those assigned to receive medical therapy (relative risk of
death, 3.9; 95 percent confidence interval, 1.9 to 9.0) (Table
2). The mortality rates during three years of follow-up are shown in Figure
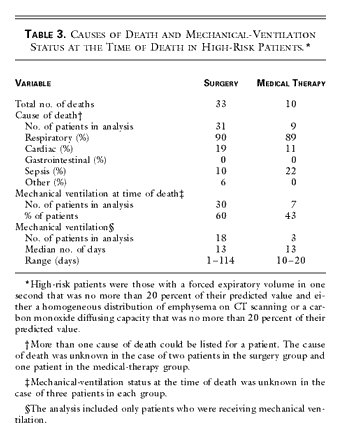
1. The cause of death was most frequently classified as respiratory: 90
percent in the case of patients in the surgery group and 89 percent in the case
of patients in the medical-therapy group. Sixty percent of surgical patients
and 43 percent of medical patients were receiving mechanical ventilation at the
time of death (Table
3). Pneumonia developed in 30 percent of the high-risk patients within 30
days postoperatively.
Although equal numbers of high-risk patients were assigned to the
two groups, more patients were assigned, by chance, to surgery early in the
trial, so that 60 such patients were included in the six-month analysis of
outcomes, as compared with 51 patients in the medical-therapy group. The
distributions of the changes from base line in the scores for functional outcomes
six months after enrollment favored neither treatment group (Figure
2). The surgery group had more deaths, but a few patients in this group had
a substantial improvement in functional status. By comparison, more patients in
the medical-therapy group were unable to undergo testing because of illness.
When the analysis was confined to survivors who completed the
six-month evaluation, the surgery group showed functional improvement in some
measures. The mean (±SD) change in exercise capacity from base line in the
surgery group was an increase of 4.5±13.0 W (measured in 34 patients), as
compared with a decrease of 4.4±14.8 W in the medical-therapy group (measured
in 23 patients) (P=0.06). The surgery group increased the distance walked in
six minutes by a mean of 14.9±63.7 m (49±209 ft) (measured in 31 patients),
whereas the medical-therapy group had a mean decrease in the distance walked of
21.6±56.7 m (71±186 ft) (measured in 24 patients) (P=0.03). Twenty-three
percent of the 31 patients in the surgery group increased the distance walked
in six minutes by more than 53.9 m (177 ft) — the minimal clinically important
difference23
— as compared with only 4 percent of the 24 patients in the medical-therapy
group (P=0.06). Patients in the surgery group had a mean increase of 5.5±6.9
percent of the predicted FEV1 (measured in 34 patients), whereas
patients in the medical-therapy group had a mean decrease of 0.4±1.9 percent
(measured in 26 patients) (P<0.001). Thirty-five percent of the 34 patients
in the surgery group had an increase in FEV1 of at least 200 ml at
six months, as compared with none of the 26 patients in the medical-therapy
group (P=0.001). The score for the Quality of Well-Being Questionnaire had
decreased by 0.01 unit in both groups at six months (P=0.94).
Discussion
This report identifies the characteristics of patients who are at
high risk for death after lung-volume–reduction surgery and who also derive
little benefit from the procedure. These patients had an FEV1 that
was no more than 20 percent of their predicted value and either homogeneous
emphysema or a carbon monoxide diffusing capacity that was no more than 20
percent of their predicted value. Within 30 days after surgery, 16 percent of
the patients in this group had died. After six months, only 33 percent of the
patients in the surgery group had an improvement in exercise capacity; 23
percent had either no change or a decrease in exercise capacity, 8 percent were
unable to complete testing, and 35 percent had died. The health-related quality
of life improved in only 28 percent of these patients, with 72 percent either
dying or having no change or a decrease in the quality of life. The
medical-therapy group had a higher percentage of poor functional outcomes but
fewer deaths.
Our analysis of functional outcomes took into account deaths and
missing data. We used this approach because studies that fail to consider
patients who have died or who are unable to complete testing can have biased
results.12 29
In our study, more surgical patients died, whereas more medical patients were
unable to perform functional testing. Patients who did not complete testing but
provided no information about why they missed the test were assigned the same
functional status as those who were known to be too ill to complete the test.
When we accounted for deaths and missing information, there was no significant
difference in the distribution of functional outcomes between groups. When we
analyzed survivors only, there was a small improvement in FEV1,
exercise capacity, and the distance walked in six minutes in the surgical
group.
Our findings have clear importance for the selection of patients
for lung-volume–reduction surgery. No single characteristic adequately defines
a group of patients for whom the surgery poses a high risk. Sensitivity
analyses using different thresholds for FEV1 and carbon monoxide
diffusing capacity and combinations of variables suggest that our criteria for
high-risk patients are nearly optimal. In selecting patients for surgery, we
used clinical tests available to community practitioners. The presence of these
characteristics should not be considered absolute contraindications to the
surgery. In borderline cases, other clinical factors, including the willingness
of the patient to accept the risk, should be used to make decisions about the
suitability of lung-volume–reduction surgery. Nonetheless, because of the
generally unfavorable outcomes, the National Emphysema Treatment Trial no
longer enrolls such patients in the clinical trial, and caution should be
exercised in performing lung-volume–reduction surgery in such patients.
Our findings are not the result of poor patient selection or a
high mortality rate at only a few centers. The high-risk patients were enrolled
at all 17 participating clinical centers, and the deaths occurred in the
surgery group at 13 of the 17 centers. Although the results of subgroup
analyses should be interpreted with caution because of the large number of
possible subgroups and the potential for false positive results, such errors
are unlikely in this analysis because of the prespecification of variables of
interest and stopping guidelines, the magnitude of the effect, and the
plausibility of the findings.
Published information identifying patients at highest risk after
lung-volume–reduction surgery is based mainly on small, uncontrolled case
series and is contradictory. Some series suggest that a very low FEV1
is associated with an increased risk of death postoperatively,30
whereas others do not.10 31 32
Some case series suggest that a very low carbon monoxide diffusing capacity
increases the risk,33 34
whereas others have not confirmed this finding.32 35 36 37 38
A recent trial of lung-volume–reduction surgery involving 48 patients stopped
enrolling participants who had a carbon monoxide diffusing capacity that was
less than 30 percent of their predicted value or who were unable to walk 150 m
(492 ft) on the shuttle-walking test, because 5 of the first 15 patients died
(3 in the surgical group and 2 in the medical group).4
The cause of the high mortality rate among patients with a low carbon monoxide
diffusing capacity may be related to impaired gas exchange. In a rabbit model
of emphysema, a reduction in the diffusing capacity was the physiological
factor that limited the amount of lung that could be removed during
lung-volume–reduction surgery.39
In patients with a low carbon monoxide diffusing capacity in association with a
low FEV1, resection of lung tissue may restrict the pulmonary
vasculature or surface area available for gas exchange enough to cause
pulmonary hypertension or worsen hypoxemia, thereby compromising survival.40
Although the presence of homogeneous emphysema is associated with
less improvement in pulmonary function after lung-volume–reduction surgery than
is the presence of heterogeneous emphysema,3 32 41 42 43 44 45 46 47 48
it has infrequently been cited as a risk factor for surgical mortality.49
In patients with homogeneous disease, lung-volume–reduction surgery involves
resection of functional lung tissue. After the removal of functional lung
tissue, patients with a very low initial FEV1 may not derive enough
benefit from the surgery to survive postoperative pulmonary complications.
Other factors such as advanced age, hypercapnia, and a low value
on the six-minute walk test increase the mortality rate associated with
lung-volume–reduction surgery. Although these characteristics are associated
with increased death rates, they did not clearly identify patients in our study
for whom surgery posed a substantially higher risk than medical treatment.
Our experience in this high-risk group of patients shows that the
increased mortality rate persists beyond the 30-day postoperative period.
Because ventilatory and circulatory support can maintain life for prolonged
periods in patients with severe physiological derangement, it is important to
assess the postoperative mortality rate for longer than 30 days and to have as
a comparison group a similar group of patients who did not undergo surgery.
Patients in both treatment groups had high rates of death from respiratory
failure, and many were receiving mechanical ventilation at the time of death.
In conclusion, we have identified a combination of physiological
and radiographic characteristics in a group of patients with emphysema that
places them at high risk of death after lung-volume–reduction surgery and who
also are unlikely to have large improvements in functional status or the
quality of life as a result of this procedure. Caution is warranted in the use
of lung-volume–reduction surgery in such patients.
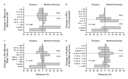
Figure 1. Kaplan–Meier Estimates of the
Probability of Death among High-Risk Patients, According to Whether They Were
Randomly Assigned to Undergo Lung-Volume–Reduction Surgery or Receive Medical
Therapy. This intention-to-treat analysis shows
the overall results for the high-risk group (Panel A), the subgroup of
patients with an FEV1 that was no more than 20 percent of their
predicted value and a homogeneous distribution of emphysema on CT scanning
(Panel B), and the subgroup of patients with an FEV1 that was no
more than 20 percent of their predicted value and a carbon monoxide diffusing
capacity that was no more than 20 percent of their predicted value (Panel C).
For each analysis the difference between groups was significant (P<0.001,
P<0.001, and P=0.005, respectively) by the log-rank test.
|
Figure 2. Changes from Base Line to the
Six-Month Follow-up Assessment in the Maximal Workload Achieved on Bicycle
Ergometry (Panel A), FEV1 (Panel B), the Distance Covered during
the Six-Minute Walk Test (Panel C), and Scores on the Quality of Well-Being
Questionnaire (Panel D) among 60 High-Risk Patients Who Were Assigned to
Undergo Lung-Volume–Reduction Surgery and 51 Who Were Assigned to Receive
Medical Therapy. The designation “Unable” indicates
patients who were too ill to complete the procedure, as well as patients who
declined to complete the procedure but who did not explain why they did not
complete the procedure. The designation “Dead” indicates patients who died
during the first six months of follow-up, even though some of these patients
had completed the six-month evaluation before death. Scores on the Quality of
Well-Being Questionnaire can range from 0 to 1, with higher scores indicating
a better quality of life. To convert values from feet to meters, divide by
3.28.
|
Table 1. Characteristics of the High-Risk
Patients at Base Line.
|
Table 2. Mortality Rates among High-Risk
Patients.
|
Table 3. Causes of Death and
Mechanical-Ventilation Status at the Time of Death in High-Risk Patients.
|
Source Information
The writing committee of the National Emphysema Treatment Trial
(Alfred Fishman, M.D., University of Pennsylvania, Philadelphia; Henry Fessler,
M.D., Johns Hopkins University, Baltimore; Fernando Martinez, M.D., University
of Michigan, Ann Arbor; Robert J. McKenna, Jr., M.D., Cedars–Sinai Medical
Center, Los Angeles; Keith Naunheim, M.D., St. Louis University, St. Louis;
Steven Piantadosi, M.D., Ph.D., Johns Hopkins University, Baltimore; Gail
Weinmann, M.D., National Heart, Lung, and Blood Institute, Bethesda, Md.; and
Robert Wise, M.D., Johns Hopkins University, Baltimore) takes responsibility
for the content of this article. Address reprint requests to Dr. Piantadosi at
the NETT Coordinating Center, 615 N. Wolfe St., Rm. 5010, Baltimore, MD 21205.
Supported by contracts with the National Heart, Lung, and Blood
Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106,
N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112,
N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119),
the Center for Medicare and Medicaid Services (formerly the Health Care
Financing Administration), and the Agency for Healthcare Research and Quality.
Footnotes
The members of the National Emphysema Treatment Trial Research
Group are listed in the Appendix.
[ Return
to Text ]
References
1. Cooper JD, Patterson GA, Sundaresan RS, et al. Results of 150
consecutive bilateral lung volume reduction procedures in patients with severe
emphysema. J Thorac Cardiovasc Surg 1996;112:1319-30.
[ Return
to Text ]
2. Criner GJ, Cordova FC, Furukawa S, et al. Prospective randomized trial
comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in
severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med
1999;160:2018-27.
[ Return
to Text ]
3. Flaherty KR, Kazerooni EA, Curtis JL, et al. Short-term and long-term
outcomes after bilateral lung volume reduction surgery: prediction by
quantitative CT. Chest 2001;119:1337-46.
[ Return
to Text ]
4. Geddes D, Davies M, Koyama H, et al. Effect of lung-volume–reduction surgery
in patients with severe emphysema. N Engl J Med 2000;343:239-45.
[ Return
to Text ] [ Abstract
]
5. Sciurba FC, Rogers RM, Keenan RJ, et al. Improvement in pulmonary function
and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J
Med 1996;334:1095-9.
[ Return
to Text ] [ Abstract
]
6. Brenner M, Yusen R, McKenna R Jr, et al. Lung volume reduction surgery for
emphysema. Chest 1996;110:205-18.
[ Return
to Text ]
7. Gelb AF, McKenna RJ Jr, Brenner M, Schein MJ, Zamel N, Fischel R. Lung
function 4 years after lung volume reduction surgery for emphysema. Chest
1999;116:1608-15.
[ Return
to Text ]
8. Pompeo E, Marino M, Nofroni I, Matteucei G, Mineo TC. Reduction pneumoplasty
versus respiratory rehabilitation in severe emphysema: a randomized study. Ann
Thorac Surg 2000;70:948-53.
[ Return
to Text ]
9. Gelb AF, McKenna RJ Jr, Brenner M. Expanding knowledge of lung volume
reduction. Chest 2001;119:1300-2.
[ Return
to Text ]
10. Argenziano M, Moazami N, Thomashow B, et al. Extended indications for lung
volume reduction surgery in advanced emphysema. Ann Thorac Surg
1996;62:1588-97.
[ Return
to Text ]
11. Butler CW, Snyder M, Wood DE, Curtis JR, Albert RK, Benditt JO.
Underestimation of mortality following lung volume reduction surgery resulting
from incomplete follow-up. Chest 2001;119:1056-60.
[ Return
to Text ]
12. Report to Congress: lung volume reduction surgery and Medicare coverage
policy: implications of recently published evidence. Baltimore: Heath Care
Financing Administration, 1998.
[ Return
to Text ]
13. National Emphysema Treatment Trial Research Group. Rationale and design of
the National Emphysema Treatment Trial (NETT): a prospective randomized trial
of lung volume reduction surgery. J Thorac Cardiovasc Surg 1999;118:518-28.
[ Return
to Text ]
14. Crapo RO, Morris AH, Gardner RM. Reference spirometric values using
techniques and equipment that meet ATS recommendations. Am Rev Respir Dis
1981;123:659-64.
[ Return
to Text ]
15. Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy
nonsmoking adults. Bull Eur Physiopathol Respir 1982;18:419-25.
[ Return
to Text ]
16. Crapo RO, Morris AH. Standardized single breath normal values for carbon
monoxide diffusing capacity. Am Rev Respir Dis 1981;123:185-9.
[ Return
to Text ]
17. American Thoracic Society. Lung function testing: selection of reference
values and interpretative strategies. Am Rev Respir Dis 1991;144:1202-18.
[ Return
to Text ]
18. American Thoracic Society. Standardization of spirometry, 1994 update. Am J
Respir Crit Care Med 1995;152:1107-36.
[ Return
to Text ]
19. American Thoracic Society. Single-breath carbon monoxide diffusing capacity
(transfer factor): recommendations for a standard technique — 1995 update. Am J
Respir Crit Care Med 1995;152:2185-98.
[ Return
to Text ]
20. Bergin C, Müller NL, Nichols DM, et al. The diagnosis of emphysema: a
computed tomographic-pathologic correlation. Am Rev Respir Dis 1986;133:541-6.
[ Return
to Text ]
21. Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in
pulmonary emphysema. Clin Radiol 1982;33:379-87.
[ Return
to Text ]
22. Bankier AA, De Maertelaer V, Keyzer C, Gevenois PA. Pulmonary emphysema:
subjective visual grading versus objective quantification with macroscopic
morphometry and thin-section CT densitometry. Radiology 1999;211:851-8.
[ Return
to Text ]
23. Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small
differences in functional status: the Six Minute Walk test in chronic lung
disease patients. Am J Respir Crit Care Med 1997;155:1278-82.
[ Return
to Text ]
24. Steele B. Timed walking tests of exercise capacity in chronic cardiopulmonary
illness. J Cardiopulm Rehabil 1996;16:25-33.
[ Return
to Text ]
25. Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as
an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis
1984;37:85-95.
[ Return
to Text ]
26. StataCorp. Stata statistical software release 7.0. College Station, Tex.:
Stata, 2001.
[ Return
to Text ]
27. Mantel N, Haenszel W. Statistical aspects of the analysis of data from
retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48.
[ Return
to Text ]
28. Lehmann EL. Nonparametrics: statistical methods based on ranks. San
Francisco: Holden-Day, 1975.
[ Return
to Text ]
29. Howard G, Chambless LE, Kronmal RA. Assessing differences in clinical
trials comparing surgical vs nonsurgical therapy: using common (statistical)
sense. JAMA 1997;278:1432-6. [Erratum, JAMA 1998;275:580.]
[ Return
to Text ]
30. Fujita RA, Barnes GB. Morbidity and mortality after thoracoscopic
pneumonoplasty. Ann Thorac Surg 1996;62:251-7.
[ Return
to Text ]
31. Eugene J, Dajee A, Kayaleh R, Gogia HS, Dos Santos C, Gazzaniga AB.
Reduction pneumoplasty for patients with a forced expiratory volume in 1 second
of 500 milliliters or less. Ann Thorac Surg 1997;63:186-90.
[ Return
to Text ]
32. McKenna RJ Jr, Brenner M, Fischel RJ, et al. Patient selection criteria for
lung volume reduction surgery. J Thorac Cardiovasc Surg 1997;114:957-64.
[ Return
to Text ]
33. Keenan RJ, Landreneau RJ, Sciurba FC, et al. Unilateral thoracoscopic
surgical approach for diffuse emphysema. J Thorac Cardiovasc Surg
1996;111:308-15.
[ Return
to Text ]
34. Hazelrigg S, Boley T, Henkle J, et al. Thoracoscopic laser bullectomy: a
prospective study with three-month results. J Thorac Cardiovasc Surg
1996;112:319-26.
[ Return
to Text ]
35. Naunheim KS, Hazelrigg SR, Kaiser LR, et al. Risk analysis for
thoracoscopic lung volume reduction: a multi-institutional experience. Eur J
Cardiothorac Surg 2000;17:673-9.
[ Return
to Text ]
36. Szekely LA, Oelberg DA, Wright C, et al. Preoperative predictors of
operative morbidity and mortality in COPD patients undergoing bilateral lung
volume reduction surgery. Chest 1997;111:550-8.
[ Return
to Text ]
37. Chatila W, Furukawa S, Criner GJ. Acute respiratory failure after lung
volume reduction surgery. Am J Respir Crit Care Med 2000;162:1292-6.
[ Return
to Text ]
38. Glaspole IN, Gabbay E, Smith JA, Rabinov M, Snell GI. Predictors of
perioperative morbidity and mortality in lung volume reduction surgery. Ann
Thorac Surg 2000;69:1711-6.
[ Return
to Text ]
39. Chen JC, Serna DL, Brenner M, et al. Diffusing capacity limitations of the
extent of lung volume reduction surgery in an animal model of emphysema. J
Thorac Cardiovasc Surg 1999;117:728-35.
[ Return
to Text ]
40. Weg IL, Rossoff L, McKeon K, Graver LM, Scharf SM. Development of pulmonary
hypertension after lung volume reduction surgery. Am J Respir Crit Care Med
1999;159:552-6.
[ Return
to Text ]
41. Gierada DS, Sloan RM, Bae KT, Yusen RD, Lefrak SS, Cooper JD. Pulmonary
emphysema: comparison of preoperative quantitative CT and physiologic index
values with clinical outcome after lung-volume reduction surgery. Radiology
1997;205:235-42.
[ Return
to Text ]
42. Maki DD, Miller WT Jr, Aronchick JM, et al. Advanced emphysema:
preoperative chest radiographic findings as predictors of outcome following
lung volume reduction surgery. Radiology 1999;212:49-55.
[ Return
to Text ]
43. Slone RM, Pilgram TK, Gierada DS, et al. Lung volume reduction surgery:
comparison of preoperative radiologic features and clinical outcome. Radiology
1997;204:685-93.
[ Return
to Text ]
44. Sugi K, Kaneda Y, Esato K. Subjective symptoms and prognosis after lung
volume reduction surgery in patients with severe pulmonary emphysema. Jpn J
Thorac Cardiovasc Surg 1999;47:489-94.
[ Return
to Text ]
45. Wisser W, Senbaklavaci O, Ozpeker C, et al. Is long-term functional outcome
after lung volume reduction surgery predictable? Eur J Cardiothorac Surg
2000;17:666-72.
[ Return
to Text ]
46. Pompeo E, Sergiacomi G, Nofroni I, Roscetti W, Simonetti G, Mineo TC.
Morphologic grading of emphysema is useful in the selection of candidates for
unilateral or bilateral reduction pneumoplasty. Eur J Cardiothorac Surg
2000;17:680-6.
[ Return
to Text ]
47. Ingenito EP, Loring SH, Moy ML, et al. Comparison of physiological and
radiological screening for lung volume reduction surgery. Am J Respir Crit Care
Med 2001;163:1068-73.
[ Return
to Text ]
48. Weder W, Thurnheer R, Stammberger U, Burge M, Russi EW, Bloch KE.
Radiologic emphysema morphology is associated with outcome after surgical lung
volume reduction. Ann Thorac Surg 1997;64:313-9.
[ Return
to Text ]
49. Hamacher J, Bloch KE, Stammberger U, et al. Two years' outcome of lung
volume reduction surgery in different morphologic emphysema types. Ann Thorac
Surg 1999;68:1792-8.
[ Return
to Text ]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.