![]()
Multivariate Analyses to Assess Treatment Effectiveness in
Advanced Head and Neck Cancer
![]() Urjeet Patel, MD; Edward Spitznagel, PhD; Jay Piccirillo, MD
Urjeet Patel, MD; Edward Spitznagel, PhD; Jay Piccirillo, MD
Objective To assess relative benefit of combined radiotherapy and surgery
over single-modality treatment for advanced-stage squamous cell carcinoma of
the aerodigestive tract by means of several multivariable analyses to control
for patient variables.
Design Medical chart review.
Setting University medical center.
Patients and
Methods The study included 532
patients receiving initial therapy between January 1, 1980, and December 31,
1989. Three multivariate techniques (multiple logistic regression, propensity
score stratification, and conjunctive consolidation) were used to compare
outcomes for treatment groups.
Main Outcome
Measure Five-year survival.
Results Survival for radiation, surgery, and combined treatment groups
were 24%, 40%, and 46%, respectively. With the use of multiple logistic
regression to control patient variables, the radiation group had a
significantly lower survival than the combined therapy group (risk ratio, 2.24;
95% confidence interval, 1.32-3.80), while there was no statistical difference
for the surgery group compared with the combined therapy group (risk ratio,
1.26; 95% confidence interval, 0.78-2.03). When analyzed by propensity score,
5-year survival was higher in each quintile for the combined therapy group than
for the group who received radiation alone (P
= .002). There was no significant difference in survival between the surgery
and combined treatment groups (P
= .25). Conjunctive consolidation was used to create a clinical staging system
to compare outcomes across treatment groups. In each clinical severity stage,
radiation alone had a lower survival than combined therapy (P = .001), while no statistical difference
was noted between surgery and combined therapy (P = .50).
Conclusions All 3 statistical techniques showed a significantly lower survival
for patients treated with radiation alone vs combined therapy. No significant
difference was noted between surgery and combined therapy. Propensity score
analysis and conjunctive consolidation are useful techniques to control
prognostic variables in cancer database studies and should be used in future
outcome studies that address more current treatment dilemmas in head and neck
oncology.
Arch Otolaryngol Head Neck
Surg. 2002;128:497-503
![]()
COMBINED SURGERY and postoperative radiotherapy
are often used to treat stages III and IV squamous cell carcinoma of the upper
aerodigestive tract.1 The choice of combined
treatment over single-modality treatment often hinges on clinical factors such
as primary site, tumor size, and extent of regional metastasis. Some centers report
improved locoregional control with combined therapy, while others report
improved survival; however, controversy exists over the benefit of combined
therapy in various clinical settings.2-4
The criterion standard for assessing the merits
of a given treatment is the prospective randomized trial. Accordingly, such
trials have long been advocated; however, these trials in head and neck cancer
treatment are inherently problematic and rare. The main problem is the
heterogeneity of the study population in terms of tumor stage, primary site,
histologic grade, age, and small sample size for any given research trial. In
addition, it is often difficult to randomize patients to treatments that are so
markedly different, as patients are hesitant to leave such grossly dissimilar
options to chance alone. Therefore, studies of treatment effectiveness in head
and neck cancer are often relegated to the realm of observational studies,
where patients are not randomized to particular treatments.
The goal of the observational study is to
measure treatment effectiveness. One of the major difficulties in the analysis
of results from observational studies is that the same clinical variables that
affect patient outcomes (age, stage, comorbidity, etc) also impact on treatment
choice.5 This may lead
to treatment selection bias. Thus, an observational study must also seek to
control selection bias to accurately measure treatment effect.
The goal of this study was to use observational
data to assess the relative benefit of combined surgery and radiotherapy over
single-modality treatment for advanced-stage squamous cell carcinoma of the upper
aerodigestive tract. To accomplish this goal, multiple statistical techniques
were used to control for selection bias.
PATIENTS AND METHODS

POPULATION UNDER STUDY
We studied 532 patients with newly diagnosed TNM stage III or IV biopsy-proved
squamous cell carcinoma who were first treated at Washington University Medical
Center, St Louis, Mo, between January 1, 1980, and December 31, 1989. These
patients were initially identified by means of records from the pathology
department of Barnes-Jewish Hospital, St Louis. Patients with American Joint
Committee on Cancer (AJCC) TNM stage III or IV squamous cell carcinoma of the
oral cavity, oropharynx, or larynx who were initially treated with
radiotherapy, surgery, or combined radiation and surgery were included in the
study population.6 Patients with
metastatic disease at the time of diagnosis were excluded from the study, as
were patients who received therapy other than the 3 above-mentioned treatment
options. Baseline and follow-up information was obtained from inpatient medical
records as well as records from the Departments of Otolaryngology and Radiation
Oncology. Full 5-year follow-up information was obtained for all 532 patients.
Supplemental date of death and death certificate information was obtained from
Equifax National Death Search (Arlington, Va).
COLLECTION OF DATA
Specially designed data extraction forms were used to ensure uniform data
collection from the medical records. Data collected from the pretreatment
interval and at the time of presentation included basic demographic
information, risk factors, medical history, symptom type and duration, complete
anatomic description of the tumor including the TNM classification with the
1992 AJCC criteria,6 pathological
description of the biopsy specimen, and details of subsequent therapy. The
zero-time for each patient was chosen as the date of first antineoplastic
intervention directed at the primary site. Follow-up data, including
development of recurrence, new primary, and subsequent treatment, were also collected.
Patient and tumor status at last follow-up or death was obtained.
CLASSIFICATION OF DATA
To maintain scientific accuracy and ensure high quality of data, imperfections
in data obtained from retrospective studies must be managed in a systematic and
consistent manner. The general methods for such management have been previously
described.7-9
SYMPTOM SEVERITY
To study the prognostic importance of symptoms for a specific cancer type, the
presence of symptoms and their relationship to the primary cancer must be
clearly established. To manage possible discrepancies in the medical record, 2
conventions were consistently applied. If a symptom was recorded by at least 1
examiner, the symptom was regarded as present. If different periods of duration
were reported, the longer duration was recorded.
The details of symptom severity staging as used
in our study have been previously described.8 Briefly, the symptoms
of dysphagia, otalgia, neck lump, and weight loss were found to be independent
predictors of survival. Accordingly, a symptom severity staging system was developed
on the basis of the presence of these symptoms. Stage was defined as none if
none of the 4 symptoms was recorded, mild if 1 of the 4 symptoms was recorded,
moderate if 2 of the 4 symptoms were recorded, and severe if 3 or 4 of the 4
symptoms were recorded.
COMORBIDITY
The presence of concomitant disease unrelated to the disease under study is
termed comorbidity. Comorbidity
has been shown to clearly impact on survival and treatment selection in several
types of cancer.10-12 The
Kaplan-Feinstein index was used to classify comorbidity for this study.13 This scheme was used
to classify the patients' comorbidity as none, mild, moderate, or severe
(grades 0, 1, 2, or 3, respectively). When a patient's condition was described
in the medical record as too sick to tolerate standard antineoplastic therapy,
a grade of 3 was assigned regardless of other illnesses. Prognostic comorbidity
was defined as grade 3, signifying the presence of concomitant illness that
significantly reduces a patient's life expectancy.
CANCER STAGING
The staging criteria for all tumors were reviewed according to the AJCC cancer
staging manual.6 All information
obtained before the zero-time was used to assess accuracy of the recorded stage
as dictated by the AJCC rules. In the case of staging discrepancies between
written notes by different physicians where the medical record lacked
sufficient anatomic information to accurately restage the tumor, the stage
assigned by the most senior otolaryngologist or radiation oncologist was
recorded. Information regarding the presence of cervical adenopathy was lacking
in 4 members of the final cohort. It was known that they had stage III or IV
disease based on T stage alone; subsequently, they were included in the study.
These members were omitted from aspects of data analysis requiring exact node
status information.
PATHOLOGICAL EXAMINATION
The histologic grade of the primary tumor was recorded from the biopsy or
primary specimen for all patients, and grades were grouped into categories of
well, moderately, and poorly differentiated. If both biopsy specimen and
primary tumor were available, the biopsy specimen was used to define the
histologic grade. Specimens graded as moderately to well differentiated were
recorded as moderate, and those graded as moderately to poorly differentiated
were recorded as poor. Histopathologic grade was absent for 4 patients; these
members were omitted from data analyses requiring pathological information.
PRIMARY TREATMENT
Information regarding each patient's initial treatment included type of treatment
(radiotherapy, surgery, or combined treatment), type of surgical procedure,
timing of radiotherapy (preoperative or postoperative), and therapeutic
complications. Subsequent treatment was defined as treatment initiated
secondary to failure of primary therapy and was also recorded.
FOLLOW-UP AND OUTCOME
Each patient was monitored for persistence, recurrence, and development of new
primary cancer. Follow-up was considered complete when either a patient's death
was documented or a minimum of 5 years' survival was obtained. The primary
outcome measure presented in this study was 5-year survival.
DATA ANALYSIS
The primary objective of data analysis was to estimate any possible benefit on
5-year survival of combined therapy over either radiation or surgery alone. The
possible benefit was estimated by 3 separate multivariable statistical
techniques: multivariate logistic regression, propensity score stratification,14 and conjunctive
consolidation.12
The information from the data extraction forms
was entered into a Paradox database (Borland International, Scotts Valley,
Calif). The specially designed database screens were equipped with internal
validity checks that facilitated reliable and efficient data entry. Periodic
review for internal consistency and comparison with separate databases was
performed to ensure accuracy of data entry. Sorting, tabulation, and
statistical analyses were performed with the SAS system, release 6.12 (SAS
Institute Inc, Cary, NC).
Logistic Regression
The impact of covariates and initial treatment options on 5-year survival was
evaluated by multiple logistic regression (PROC LOGIST function). The logistic
regression modeled the dependent variable of 5-year survival from the
independent patient, tumor, and treatment variables. A regression model was fit
with the use of the following covariates: age group, sex, race, prognostic comorbidity,
symptom severity, pathological findings, tumor size, presence of adenopathy,
primary site, and initial treatment choice. Of the 532 patients, 7 were
eliminated from the regression model because of missing information as
described in the "Cancer Staging" and "Pathological
Examination" subsections. The multivariable regression had an area under
the receiver operating characteristic curve of 0.72. This means that the
regression model was fairly accurate in discriminating survivors from nonsurvivors
on the basis of covariate information. Adjusted risk ratios and corresponding
95% confidence intervals and P
values were obtained according to reference groups for each variable.
Propensity Score
The goal of propensity analysis is to reduce the effect of selection bias
between 2 treatment options as described by Rosenbaum and Rubin.15-17 Selection bias is
clearly problematic in observational studies when clinical covariates (age,
comorbidity, tumor stage, etc) impact on both treatment (radiation, surgery, or
combined therapy) and outcome (5-year survival). Propensity score
stratification seeks to replace the wide host of confounding covariates that
may be present in an observational study with a single variable function of
these covariates. The covariates are summarized into a single probability
function called the propensity score
that describes the likelihood of receiving treatment A (surgery plus radiation,
for example) vs treatment B (radiation alone). The propensity score can be
estimated through logistic regression of the covariates on treatment choice.
Accordingly, each individual has a propensity score that represents the
probability of being treated with combined therapy rather than radiation alone.
The propensity score is then used in further analysis as the single confounding
variable.
The study population is then stratified into a
discrete number of groups, usually 5, on the basis of the propensity score.
Stratification into 5 quintiles has been shown by Rosenbaum18 to eliminate more
than 90% of selection bias by covariates. Within each propensity stratum, there
will generally be a number of patients who received combined therapy or
radiation alone. The rationale behind the propensity score scheme is as
follows: If 2 patients have the same propensity score, then it follows that
they have the same likelihood of receiving combined treatment as radiation alone
on the basis of their given covariates. If the 2 patients receive different
treatments, then the choice of treatment can be considered random. The same
principle holds for 2 groups with similar propensity scores. Within a given
propensity stratum, the group of patients receiving combined therapy will have
a distribution of propensity scores similar to that of patients who received
surgery alone. Subsequently, the patients composing one treatment group can be
considered to be randomly chosen from the entire propensity stratum with regard
to their confounding covariate data. Within a propensity stratum, the
multivariate distribution of covariates should differ only randomly between the
2 treatment groups as if they had been randomly assigned a treatment option.
Thus, use of this technique with stratification into quintiles eliminates
selection bias between 2 treatment groups.15
In our study, propensity score analysis was
first used to assess treatment effect between patients receiving combined
therapy vs radiation alone. To identify variables that were unbalanced between
the 2 treatment groups, bivariate screening was performed for all potential
confounding covariates that potentially impact on treatment decision. A
multiple logistic regression was performed in a stepwise fashion to determine
important predictors of treatment selection. A logistic regression model was
then fit with variables found to be significant (P<.15) in the logistic analysis. The area under the
receiver operating characteristic curve for this regression model was 0.76,
indicating good discrimination between patients receiving combined vs single
therapy. With this model, a propensity score was calculated for each patient
that predicts the likelihood of being initially treated with combined surgery
and radiation therapy vs radiation alone.19 Patients were then
sorted by propensity score and clustered into quintiles accordingly. Bivariate
screening and logistic regression were then performed within each quintile to
identify any remaining bias among covariates after stratification by propensity
score. The effect of treatment assignment on 5-year survival was then analyzed
within each quintile. The Mantel-Haenszel odds ratio was calculated in addition
to the Cochran-Mantel-Haenszel (CMH) ![]() 2. The Mantel-Haenszel odds ratio represents a
composite of the 5 odds ratios derived from each quintile, and the CMH
2. The Mantel-Haenszel odds ratio represents a
composite of the 5 odds ratios derived from each quintile, and the CMH ![]() 2
reflects the statistical significance of the odds ratio. The Breslow-Day test,
which indicates whether there is homogeneity of the odds ratios among the 5
quintiles, was also performed.20
2
reflects the statistical significance of the odds ratio. The Breslow-Day test,
which indicates whether there is homogeneity of the odds ratios among the 5
quintiles, was also performed.20
The same process described above was then
repeated for the comparison of combined therapy vs surgery alone. A similar
regression model was fit with significant covariates. The area under the
receiver operating characteristic curve for this model was 0.67, indicating
good discrimination of treatment options. Similar analysis to estimate the
effect of treatment assignment was then performed after estimation of the
propensity scores and stratification into propensity quintiles.
Conjunctive Consolidation
An alternative multivariable technique produces clusters of patients through
conjunctive consolidation and is exemplified by the TNM staging systems.12 Use of conjunctive
consolidation to evaluate treatment effect has been previously described.12 Under this system,
patients are stratified according to values of the prognostic covariates (such
as TNM, age, comorbidity, etc). With the addition of each new variable, the
number of strata grows and the number of patients within each stratum
decreases. The problem with addition of numerous variables is the exponential
growth in the number of stratified groups of patients, making further analysis
of patients within each group difficult. Through conjunctive consolidation,
groups of categories are clustered by means of unions and intersections of
Boolean algebra.12 This process is the
cross-table analysis of the conjoined effect of 2 variables on the outcome of
interest. Each conjoined cell contains patients with similar values for the 2
variables being conjoined. Adjacent cells can then be combined according to
clinical and statistical similarity. This allows for the inclusion of numerous
clinical factors while avoiding the subsequent increase in number of
categories.
To assemble data for evaluating the prognostic
covariates, patients were combined according to a therapeutic "nil
hypothesis," as previously described.12 This makes a
tentative clinical assumption that treatment had no effect on a patient's
clinical course. With this assumption made, the data were combined for all
patients regardless of treatment. Patients were then categorized according to
their nontherapeutic prognostic covariates, and survival outcomes were then
analyzed for each category. After prognostic factors were consolidated
according to the nil hypothesis, the impact of different treatment options was
explored for patients within given stages.
In the present study, the technique of
conjunctive consolidation was first applied to combine 2 clinical variables,
age group and prognostic comorbidity, into a 3-category composite functional
staging system. Patients younger than 55 years with no comorbidity were
categorized as stage ![]() , those aged 55
to 69 years with no comorbidity as stage
, those aged 55
to 69 years with no comorbidity as stage ![]() ,
and those older than 69 years or with prognostic comorbidity as stage
,
and those older than 69 years or with prognostic comorbidity as stage ![]() . The cancer
variables of tumor size and presence of adenopathy were then combined to form a
composite tumor staging system (1, 2, and 3). Patients with T3,4 N0 disease
were combined with patients with T1 N1 disease into cancer stage 1; T2 N1 was
classified as stage 2; and T3,4 N1 was categorized as stage 3. The functional
stage and the tumor stage were next combined to create a composite clinical
severity staging system (A, B, C, and D). Stage
. The cancer
variables of tumor size and presence of adenopathy were then combined to form a
composite tumor staging system (1, 2, and 3). Patients with T3,4 N0 disease
were combined with patients with T1 N1 disease into cancer stage 1; T2 N1 was
classified as stage 2; and T3,4 N1 was categorized as stage 3. The functional
stage and the tumor stage were next combined to create a composite clinical
severity staging system (A, B, C, and D). Stage ![]() 1 was classified as composite stage A. Stages
1 was classified as composite stage A. Stages ![]() 2 and
2 and ![]() 1 were combined
into composite stage B. Stages
1 were combined
into composite stage B. Stages ![]() 3,
3, ![]() 2,
2, ![]() 3,
3, ![]() 1, and
1, and ![]() 2 were combined
into stage C. Finally, stage
2 were combined
into stage C. Finally, stage ![]() 3 was
classified as stage D. With the use of conjunctive consolidation in this
manner, the 4 covariates of age, comorbidity, tumor size, and cervical
adenopathy were conjoined into a 4-category composite clinical severity staging
system.
3 was
classified as stage D. With the use of conjunctive consolidation in this
manner, the 4 covariates of age, comorbidity, tumor size, and cervical
adenopathy were conjoined into a 4-category composite clinical severity staging
system.
The association between clinical severity stage
and 5-year survival was examined. There was a strong relationship that was both
clinically impressive and statistically significant. The ![]() 2 for linear trend was P
= .001. Next, treatment effect was examined within composite clinical severity
stage groups. Patients were grouped according to initial treatment within
composite stages. Survival rates were calculated for each treatment group by
composite stage. A
2 for linear trend was P
= .001. Next, treatment effect was examined within composite clinical severity
stage groups. Patients were grouped according to initial treatment within
composite stages. Survival rates were calculated for each treatment group by
composite stage. A ![]() 2 test
of significance was performed by comparing survival rates across the different
therapies within composite clinical severity staging groups. The
Mantel-Haenszel odds ratio was then calculated for each single modality
treatment vs combined therapy in addition to the CMH
2 test
of significance was performed by comparing survival rates across the different
therapies within composite clinical severity staging groups. The
Mantel-Haenszel odds ratio was then calculated for each single modality
treatment vs combined therapy in addition to the CMH ![]() 2. The Breslow-Day test was also performed to verify
that the odds ratios derived from each stage were homogeneous.
2. The Breslow-Day test was also performed to verify
that the odds ratios derived from each stage were homogeneous.
RESULTS

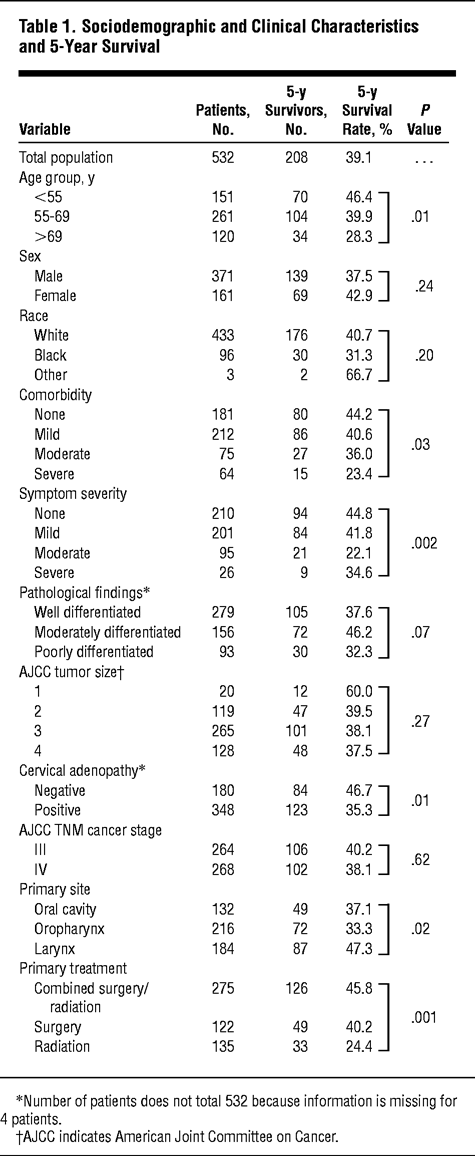
The characteristics of the 532 study patients
are presented in Table 1.
The study population was 70% male, and more than 75% were white. Most patients
had either absent or mild comorbidity as well as symptom severity. More than
65% had evidence of cervical adenopathy and were equally divided between TNM
stage III and stage IV disease. Disease was most prevalent in the oropharynx
(40%), followed by the larynx (35%) and then the oral cavity (25%). The most
common initial treatment plan was combined surgery plus radiation (52%),
followed by radiation alone (25%) and then surgery alone (23%).
The relationship between covariates, including
treatment and survival, is also shown in Table 1.
Patient characteristics that were associated with decreased survival included
increasing age (P = .01),
increasing comorbidity (P = .03),
and increasing symptom severity (P
= .002). Presence of cervical adenopathy was associated with a significant
decrease in survival from 47% to 35% (P
= .01). Laryngeal cancer was associated with the highest 5-year survival at
47%, compared with oral cavity and oropharyngeal disease at 37% and 33%,
respectively. Looking at treatment, radiation alone was associated with
significantly lower survival of 24% while survival rates for combined therapy
and surgery alone were 46% and 40%, respectively.
Multivariate logistic regression was used to
assess the impact of covariates and treatment on survival. Table 2
shows the adjusted risk ratios for covariates and treatment for the study population.
Increasing age and male sex were both related to a decreased 5-year survival
rate. Similarly, the presence of cervical adenopathy and tumor size greater
than stage 1 impact negatively on survival. With regard to primary site,
patients with cancer of the oral cavity and oropharynx had significantly higher
risk of death than the patients with laryngeal cancer. With regard to
treatment, the group of patients treated with radiation alone had a
significantly higher risk of death than those receiving combined therapy (risk
ratio, 2.24; 95% confidence interval, 1.32-3.80). While treatment with surgery
alone did reflect an increased risk of death compared with patients receiving
combined treatment, this risk was not found to be statistically significant (risk
ratio, 1.26; 95% confidence interval, 0.78-2.03).
Stratification by propensity score and
assessment of the treatment effect of each single-modality treatment and
combined therapy on survival were performed. The population receiving either
radiation alone or combined therapy was examined first (n = 410). The
population was stratified into propensity quintiles as previously described. Table 3
shows survival rates for both treatment groups after stratification. The
percentage of patients receiving combined therapy decreased from the first
propensity quintile to the fifth as predicted by the propensity model. In each
of the 5 strata, patients receiving combined therapy had a higher 5-year
survival rate than the group receiving radiation alone. In quintiles 1 and 3,
the difference in survival was statistically significant. The P value for the CMH ![]() 2 comparing survival between the treatment groups while
controlling for propensity quintile was .002, suggesting a strong difference in
survival between those receiving radiation alone vs combined treatment.
2 comparing survival between the treatment groups while
controlling for propensity quintile was .002, suggesting a strong difference in
survival between those receiving radiation alone vs combined treatment.
Propensity score analysis was similarly
performed for patients initially receiving either surgery alone or combined
therapy (n = 397). Table 4
shows survival rates for these treatment groups after propensity score
stratification. As expected, the percentage of patients receiving combined
therapy decreased from the first to the fifth quintile. Within quintile 2,
patients receiving surgery alone had a higher survival than those receiving
combined treatment, while in the remaining quintiles, patients with combined
treatment had more favorable survival. In none of the quintiles was the
difference in survival statistically significant. The P value for the CMH ![]() 2 was .25, suggesting no significant difference between
treatment groups across quintiles.
2 was .25, suggesting no significant difference between
treatment groups across quintiles.
Conjunctive consolidation was performed as
previously described. Table 5
shows survival rates for all patients according to composite stage (A, B, C, or
D). A prognostic gradient was noted in survival from stage A through stage D,
with a significant ![]() 2 for
linear trend (P = .001). Patients
were separated into treatment group, and survival rates were then compared
within composite staging groups (Table 6).
In all 4 composite staging groups, survival rates were higher for patients
receiving combined therapy compared with radiation alone, with a statistically
significant difference noted in 3 of the 4 stages. The CMH
2 for
linear trend (P = .001). Patients
were separated into treatment group, and survival rates were then compared
within composite staging groups (Table 6).
In all 4 composite staging groups, survival rates were higher for patients
receiving combined therapy compared with radiation alone, with a statistically
significant difference noted in 3 of the 4 stages. The CMH ![]() 2 was highly significant (P = .001). Comparing groups receiving
combined therapy vs surgery alone, survival was higher for combined therapy in
stages A, B, and D, while surgery alone was favored for stage C patients. In
only stage D was there a statistically significant difference in survival
between patients receiving surgery vs combined treatment. No statistical
difference was noted between the 2 groups by the CMH
2 was highly significant (P = .001). Comparing groups receiving
combined therapy vs surgery alone, survival was higher for combined therapy in
stages A, B, and D, while surgery alone was favored for stage C patients. In
only stage D was there a statistically significant difference in survival
between patients receiving surgery vs combined treatment. No statistical
difference was noted between the 2 groups by the CMH ![]() 2 (P =
.50).
2 (P =
.50).
COMMENT

Our research demonstrates the usefulness of
multivariate analysis with regard to head and neck oncology observational
studies. The relative benefit of combined therapy over single-modality therapy
was assessed by means of multiple logistic regression, propensity score
analysis, and conjunctive consolidation. The 3 forms of analysis concurred in
their findings that combined therapy offered significantly higher survival at 5
years than radiotherapy alone. In contrast, no significant difference was seen
when combined therapy was compared with surgical treatment alone. By using
multivariate analysis to eliminate selection bias, the difference in survival
can be attributed to treatment effect without the influence of confounding
variables.
Previous studies have compared combined therapy
with single-modality treatment for various tumors in the head and neck region.21-24 While many of
these studies seek to measure treatment effect, few of them compare different
treatment options while controlling for selection bias. Patient variables such
as comorbidity, pathological grade, symptom severity, and age are often omitted
from analysis despite the fact that these variables may influence treatment
choice as well as outcome. Subsequently, conclusions are drawn regarding
treatment effectiveness without adequately controlling for potential selection
bias. Without such control, the conclusions may not accurately assess true
treatment effectiveness.
While multivariate analysis does permit a more
controlled estimate of treatment effectiveness, there do exist potential
inaccuracies in its formulation. Each multivariate model is able to control
only the study variables included in the analysis. In our study, there was no
variable to quantify the amount of radiotherapy given to each patient, nor was
there any measure in the quality of the surgery performed. Subsequently, it is
possible that variables exist that would alter the measured treatment effects
had they been included in the multivariate analysis. In addition, a given
multivariate analysis makes use of statistical models to approximate the data
being analyzed. The degree to which a given model fits the data appropriately
can vary and needs to be considered when the results of statisitical analysis
are interpreted. This is especially true when different statistical tools yield
varying results when the same data are analyzed.
Multivariate analysis is a highly useful tool to
measure treatment effect in observational studies. Multiple logistic regression
is a statistical technique that is frequently used in analysis of observational
study results. Propensity score analysis and conjunctive consolidation are also
highly effective at controlling selection bias to measure treatment
effectiveness. The use of these techniques will improve the ability to
accurately measure treatment effectiveness in observational studies. These
tools may be applied to more current clinical dilemmas, such as chemoradiation
protocols compared with surgical resection for treatment of head and neck
cancer.
Author/Article Information
![]()
From the Departments of Otolaryngology (Drs Patel and Piccirillo) and
Mathematics (Dr Spitznagel), Washington University, St Louis, Mo.
Corresponding author and reprints: Jay Piccirillo, MD, Department of Otolaryngology,
660 S Euclid St, Box 8115, St Louis, MO 63110 (e-mail: [log in to unmask]).
Accepted for publication October 11, 2001.
This study was supported in part by grant
R01CA2072 from the National Cancer Institute, Bethesda, Md (Dr Piccirillo).
This study was presented at the Fifth
International Conference on Head and Neck Cancer, San Francisco, Calif, July
31, 2000.
REFERENCES

1.
Forastiere A, Goepfert H, Goffinet D, et al, and the National Comprehensive
Cancer Network.
NCCN practice guidelines for head and neck cancer.
Oncology (Huntingt).
1998;12:39-147.
MEDLINE
2.
El Badawi SA, Goepfert H, Fletcher GH, Herson J, Oswald MJ.
Squamous cell carcinoma of the pyriform sinus.
Laryngoscope.
1982;92:357-364.
MEDLINE
3.
Kirchner JA, Owen JR.
Five hundred cancers of the larynx and pyriform sinus: results of treatment by
radiation and surgery.
Laryngoscope.
1977;87:1288-1303.
MEDLINE
4.
Van den Bogaert W, Ostyn F, van der Schueren E.
Hypopharyngeal cancer.
Radiother Oncol.
1985;3:311-318.
MEDLINE
5.
Horwitz RI.
The experimental paradigm and observational studies of cause-effect
relationships in clinical medicine.
J Chronic Dis.
1987;40:91-99.
MEDLINE
6.
American Joint Committee on Cancer.
Manual for Staging of Cancer.
Philadelphia, Pa: JB Lippincott Co; 1992.
7.
Feinstein AR, Pritchett JA, Schimpff CR.
The epidemiology of cancer therapy, II.
Arch Intern Med.
1969;123:323-344.
MEDLINE
8.
Feinstein AR, Pritchett JA, Schimpff CR.
The epidemiology of cancer therapy, III: the management of imperfect data.
Arch Intern Med.
1969;123:448-461.
MEDLINE
9.
Feinstein AR.
Taxonorics, II.
Arch Intern Med.
1970;126:1053-1067.
MEDLINE
10.
Piccirillo JF, Wells CK, Sasaki CT, Feinstein AR.
New clinical severity staging system for cancer of the larynx.
Ann Otol Rhinol Laryngol.
1994;103:83-92.
MEDLINE
11.
Feinstein AR, Schimpff CR, Andrews JFJ, Wells CK.
Cancer of the larynx.
J Chronic Dis.
1977;30:277-305.
MEDLINE
12.
Feinstein AR, Wells CK.
A clinical-severity staging system for patients with lung cancer.
Medicine (Baltimore).
1990;69:1-33.
MEDLINE
13.
Kaplan MH, Feinstein AR.
The importance of classifying initial co-morbidity in evaluating the outcome of
diabetes mellitus.
J Chronic Dis.
1974;27:387-404.
MEDLINE
14.
Rosenbaum PR, Rubin DB.
The central role of the propensity score in observational studies for causal
effects.
Biometrics.
1983;70:41-55.
15.
Rosenbaum PR, Rubin DB.
Reducing bias in observational studies using subclassification on the
propensity score.
J Am Stat Assoc.
1984;79:516-524.
16.
Rubin DB.
Tasks in statistical inference for studying variation in medicine.
Med Care.
1993;31:YS103-YS110.
MEDLINE
17.
Rubin DB.
Estimating causal effects from large data sets using propensity scores.
Ann Intern Med.
1997;127:757-763.
MEDLINE
18.
Rosenbaum PR.
Quantiles in nonrandom samples and observational studies.
J Am Stat Assoc.
1995;90:1424-1431.
19.
D'Agostino RBJ.
Propensity score methods for bias reduction in the comparison of a treatment to
a non-randomized control group.
Stat Med.
1998;17:2265-2281.
MEDLINE
20.
Breslow NE, Day NE.
The Analysis of Case-Control Studies.
Lyon, France: IARC Scientific Publications; 1980. Breslow NE, Day NE, eds. Statistical Methods in Cancer Research;
vol 1.
21.
Nisi KW, Foote RL, Bonner JA, McCaffrey TV.
Adjuvant radiotherapy for squamous cell carcinoma of the tongue base.
Int J Radiat Oncol Biol Phys.
1998;41:371-377.
MEDLINE
22.
Zelefsky MJ, Kraus DH, Pfister DG, et al.
Combined chemotherapy and radiotherapy versus surgery and postoperative
radiotherapy for advanced hypopharyngeal cancer.
Head Neck.
1996;18:405-411.
MEDLINE
23.
Gujrathi D, Kerr P, Anderson B, Nason R.
Treatment outcome of squamous cell carcinoma of the oral tongue.
J Otolaryngol.
1996;25:145-149.
MEDLINE
24.
Simpson D, Robertson AG, Lamont D.
A comparison of radiotherapy and surgery as primary treatment in the management
of T3 N0 M0 glottic tumours.
J Laryngol Otol.
1993;107:912-915.
MEDLINE
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.