|
Azithromycin
for acute bronchitis: a randomised, double-blind, controlled trial |
Lancet 2002; 359: 1648-54
Arthur T Evans, Shahid Husain,
Lakshmi Durairaj, Laura S Sadowski, Marjorie Charles-Damte, Yue Wang
Collaborative Research Unit, Department of Medicine, Cook
County Hospital and Rush Medical College, Chicago, IL (A T Evans MD, L S Sadowski MD, M Charles-Damte
RN, Y Wang PhD); Division of Infectious
Diseases, University of Pittsburgh Medical Center, Pittsburgh, PA (S
Husain MD); and Division of Pulmonary,
Critical Care and Occupational Medicine, University of Iowa College of
Medicine, Iowa City, IA, USA (L Durairaj MD)
Correspondence to: Dr Arthur T Evans, Collaborative Research Unit,
Cook County Hospital, Room 1600, Administration Building, 1900 W Polk St,
Chicago, IL 60612, USA (e-mail:[log in to unmask])
Summary
Introduction
Methods
Results
Discussion
References
|
Summary
|
Background The value of azithromycin for treatment of acute bronchitis is
unknown, even though this drug is commonly prescribed. We have investigated
this question in a randomised, double-blind, controlled trial.
Methods Adults diagnosed with acute bronchitis, without evidence of
underlying lung disease, were randomly assigned azithromycin (n=112) or vitamin
C (n=108) for 5 days (total dose for each 1·5 g). All individuals were also
given liquid dextromethorphan and albuterol inhaler with a spacer. The primary
outcome was improvement in health-related quality of life at 7 days; an
important difference was defined as 0·5 or greater. Analysis was by intention
to treat.
Findings The study was stopped by the data-monitoring and safety committee
when 220 patients had been recruited. On day 7, the adjusted difference in
health-related quality of life was small and not significant (difference 0·03
[95% CI -0·20 to 0·26], p=0·8). 86 (89%) of 97 patients in the azithromycin
group and 82 (89%) of 92 in the vitamin C group had returned to their usual
activities by day 7 (difference 0·5% [-10% to 9%], p>0·9). There were no
differences in the frequency of adverse effects; three patients in the vitamin
C group discontinued the study medicine because of perceived adverse effects,
compared with none in the azithromycin group. Most patients (81%) reported
benefit from the albuterol inhaler.
Interpretation Azithromycin is no better than low-dose vitamin C for acute
bronchitis. Further studies are needed to identify the best treatment for this
disorder.
|
Introduction
|
Every year, more than ten million US
adults visit physicians for acute bronchitis, and most of them receive
antibiotics.1,2 Many experts condemn such treatment, citing three
main reasons: weak or conflicting experimental evidence of clinical benefit,
lack of a strong biological rationale (the causative pathogens are viruses in
most cases), and increasing societal concern about widespread antibiotic
resistance.2-5
Nine randomised controlled trials of
three different antibiotics for acute bronchitis have been published;6-14
four demonstrated clinical benefit.9-12 Meta-analyses of these
studies15-18 have been beset by fundamental problems:2,15-18
different studies measured different outcomes; the reliability and validity of
outcome measurement was uncertain; and some outcomes had questionable clinical
significance (for example, duration of purulent sputum). Equally important, no
published study has measured the effect of antibiotic therapy on quality of
life, although four studies8-11 reported on limitations in work or
other activities.18 Finally, no study has assessed any of the newer
antibiotics widely promoted and prescribed today, including quinolones and the
newer macrolides.
Azithromycin is a macrolide antibiotic
commonly prescribed for acute bronchitis. It has a broad spectrum of activity
and infrequent adverse effects, and it is easy to take (once daily for 5 days).
However, it is also expensive, and the only published evidence of its efficacy
in acute bronchitis comes from several small equivalence studies19-22
and one large, open, uncontrolled, drug-company-sponsored case series.23,24
In a randomised, double-blind, controlled
clinical trial, we tested whether patients prescribed azithromycin for acute
bronchitis returned to work (or other usual activities) sooner and whether they
experienced greater improvements in health-related quality of life than
patients prescribed low-dose vitamin C.
![]()
|
Methods
|
|
Participants |
After approval of the study protocol by
the institutional review board, we recruited adult patients without chronic
lung disease who presented with cough of 2-14 days duration (with or without
sputum production) and were diagnosed with acute bronchitis by attending
physicians in the adult ambulatory screening clinic of Cook County Hospital,
Chicago, Illinois, USA, during weekdays from December, 1999, until March, 2000.
Patients were enrolled after giving written informed consent. Reasons for
exclusion were: pregnancy; other infectious diseases necessitating
antimicrobial therapy; chronic lung disease (including asthma) or current
treatment with bronchodilators or glucocorticoids;
angiotensin-converting-enzyme inhibitor started within the previous 4 weeks;
antibiotic treatment within the previous 2 weeks; need for hospital admission;
allergies to macrolides, bronchodilators, or vitamin C; duration of cough less
than 2 days or longer than 2 weeks; or any clinical characteristic suggesting
pneumonia, including oral temperature above 38·9°C (102°F), respiratory rate
greater than 25 per min, or infiltrates or other abnormalities on chest
radiograph.
Immediately before the study, physicians
who would be seeing patients with cough in the ambulatory screening clinic
during the study period took a 2-h training course that covered the assessment
of patients presenting with acute cough, the diagnosis of acute bronchitis, the
evidence supporting treatment options for cough25 and acute
bronchitis, and the rationale for the planned study. The physicians were
reminded that the diagnosis of acute bronchitis is largely one of exclusion,
and therefore other diagnoses should be carefully considered--such as
pneumonia, viral upper-respiratory-tract illness, influenza, gastro-oesophageal
reflux, sinusitis, postnasal drip, heart failure, and asthma--before a final
diagnosis of acute bronchitis is made. All laboratory and radiographic studies
were left to the discretion of the treating physicians, but if a chest
radiograph was done before enrolment, any pulmonary abnormality was grounds for
exclusion.
|
Design and procedures |
Patients were randomly assigned one of
two study drugs, azithromycin (total dose 1·5 g) or vitamin C (total dose 1·5
g). All study drugs were prepared at one site by an independent pharmaceutical
consultant (MediDerm, Chicago, IL, USA) in opaque sealed capsules that were
identical in appearance, taste, and smell. Six capsules were packaged in
bottles that were identical except for labels that differed only by a unique
identification number. Each azithromycin capsule contained the equivalent of a
250 mg caplet. Each vitamin C capsule contained 250 mg vitamin C and enough
dextrose to fill the capsule to a volume equivalent to the azithromycin
capsule.
A research associate, not a member of the
research team, prepared the randomisation code using a computer-generated
(Arcus QuickStat Biomedical, Cambridge, UK) random allocation schedule in three
unequal blocks. The external pharmaceutical company then sealed the study drugs
in sequentially numbered identical containers according to the allocation
sequence. All members of the research team--investigators, project coordinator,
physicians, patient educators, research assistants, data collectors, data-entry
staff, and the research pharmacist who dispensed all study drugs in
sequence--were unaware of the allocation schedule. Masking was maintained until
data analysis except on two occasions, when physicians specifically requested
knowledge of the study drug because of patients' lack of improvement.
Before leaving the clinic, each patient
was directly observed taking two study drug capsules. Participants were then
instructed to take one study drug capsule in the morning on an empty stomach 1
h before eating breakfast, for the next 4 days (ie, a total of six capsules).
We chose vitamin C instead of a
traditional placebo compound, such as dextrose, because the results of several
focus groups we undertook a year earlier with members of the target population
showed that they would probably refuse to take part in any randomised trial of
acute bronchitis if the placebo group received a "sugar pill".
However, they suggested that a randomised trial of antibiotic and a
multivitamin or some other vitamin pill would be acceptable. We followed this
recommendation and used vitamin C as the comparison drug, since it can be
administered in a capsule without any identifying taste or smell and because
there is no evidence of efficacy in acute bronchitis or any other respiratory
illness at the doses given.26
Patients in both treatment groups all
received aggressive standard symptomatic therapy of proven benefit. All
participants were given 240 mL (8 ounces) of dextromethorphan syrup and were
instructed to take 10 mL by mouth every 6 h as necessary for cough during the
day and 15 mL at bedtime.25 All participants also received one
albuterol inhaler with a spacer and were instructed to inhale two puffs with
the spacer every 6 h as necessary for cough.27,28 A research nurse
or trained research assistant gave instructions to all participants on taking
all medications and provided supervised practice in use of the inhaler and
spacer. Patients were instructed not to take any other medications for their
illness during the study period.
The primary endpoint was health-related
quality of life on day 7 of follow-up. Secondary endpoints were return to usual
daily activities at follow-up, domain scores that comprise health-related
quality of life, and adverse effects.
On enrolment, trained research assistants
interviewed study participants to establish baseline measurements of
health-related quality of life and to describe the activity limitations
attributed to the symptoms of acute bronchitis. In addition, the examining
physicians completed a standard data-collection instrument for recording
clinical data from their history, physical examination, clinical assessment,
and any tests done.
On day 3 (about 48 h after enrolment), a
research assistant telephoned all participants for a follow-up interview to
inquire whether the patient had returned to his or her usual activities at
work, home, or school and to reassess the patient's health-related quality of
life. In addition, patients were interviewed about use of all medications and
any possible adverse effects. On day 7, the interview was repeated, with
additional questions included at the end to assess the adequacy of masking and
patients' subjective impressions about the effectiveness of all medications. If
a patient was not available for interview on day 7 at the scheduled time, the
interviewers attempted to contact him or her up to six times daily for 3 consecutive
days before declaring the patient lost to follow-up.
The acute bronchitis health-related
quality-of-life interview was adapted from similar instruments developed at
McMaster University to measure changes in health-related quality of life for patients
treated for asthma,29 rhinitis,30 chronic lung disease,31
or congestive heart failure.32 A score is obtained by taking the
mean of 22 equally weighted items representing four domains: effects on daily
activities (three activities specified by the individual and three general
activities--sleep, recreational activities, and regular activities at home and
at work); effects of coughing, including chest pain and dyspnoea (eight items);
general symptoms (four items); and emotional functioning (four items; panel).
For each item, patients are asked to indicate on a 7-point scale how troubled
they have been during the previous few days as a result of their bronchitis
symptoms, from not troubled at all (0) to extremely troubled (6).
We assessed the adequacy of masking by
asking all participants at the day 7 interview to guess whether they received
azithromycin or vitamin C.
|
Statistical analysis |
We used multivariate ANCOVA with repeated
measures to assess the effect of treatment group on health-related
quality-of-life scores during the follow-up period, while controlling for any
differences at baseline. In addition, we used the simpler ANCOVA to assess
differences between groups on day 3 and day 7, separately.
A difference between groups of 0·5 points
on the quality-of-life scale was selected as the smallest important difference
based on published research of similar health-related quality-of-life scales.33-36
However, the previous research that identified 0·5 as a reasonable
"smallest clinically important difference" involved chronic
illnesses--allergic rhinitis, asthma, congestive heart failure, chronic
obstructive lung disease--in which a small difference over a long period might
be judged important. For self-limited illnesses of shorter duration, such as
acute bronchitis, the smallest important difference might therefore be greater
than 0·5. A difference of 0·5 points represents a change, for example, from 2
(somewhat troubled) to 1 (hardly troubled at all) for half of the symptoms or
activities addressed in the questionnaire (on the assumption the remaining
items are unchanged).
In analysing secondary endpoints, we
compared the proportion of participants who had returned to their usual daily
activities on day 3 and day 7 in the azithromycin and vitamin C groups. We used
*2 or Fisher's exact tests and constructed near-exact 95% CI for the
difference in proportions by the method of Miettinen.37
We had originally planned for a sample
size of 400 patients so that we would have sufficient power to test treatment
effects within several clinical subgroups that previous research had suggested
as potentially important (based on age, duration of cough, magnitude of fever,
and severity of symptoms). However, an interim analysis was done at the end of
the fourth month to help decide whether to continue recruiting. The
data-monitoring and safety committee, independent of the investigators, was
presented with interim analyses addressing primary and secondary endpoints, as
well as adverse effects, after the first 220 patients had been enrolled.
Unaware of treatment-group identity, the committee concluded that the study
should stop enrolment because the outcomes were equivalent and there was
sufficient precision to be confident that the likelihood of detecting a
clinically meaningful difference with a larger sample was so small that
continued enrolment of patients would be inappropriate.
The sample size of 220 provided power of
95-99% to detect a difference between the groups of 0·5 points in
health-related quality of life, on the assumption of a population SD of 0·8-1·0
points and ![]() =0·05. This sample size also
provided 80% power to detect a difference of 10% in the proportion who had
returned to their usual activities by day 7.
=0·05. This sample size also
provided 80% power to detect a difference of 10% in the proportion who had
returned to their usual activities by day 7.
Statistical analyses were done with SPSS
(version 10), Stata (version 6), and Arcus Quickstat Biomedical (version 1). We
updated a previously published meta-analysis18 using the
DerSimonian-Laird random-effects model for risk differences (Arcus Quickstat
Biomedical). This meta-analysis model was used instead of other methods that
pool odds ratios18 because the results have a more transparent
clinical interpretation and because the study by Williamson and colleagues11
can be included in the analysis, despite an odds ratio that is undefined.
![]()
|
Results
|
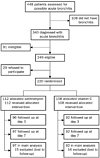
Of the 340 patients who met the inclusion
criteria, 91 were ineligible because of one or more exclusion criteria and 29
declined to participate; we enrolled the remaining 220 patients (figure 1).
Table 1 shows the baseline demographic and clinical characteristics of study
participants. 93 (96%) patients in the azithromycin group and 87 (95%) in the
vitamin C group reported taking at least five of the six study drug capsules
(p=0·7).
Figure 1: Trial profile |
|
Characteristic |
Azithromycin
|
Vitamin
C |
|
|
(n=112) |
(n=108) |
|
Demography |
|
|
|
Mean (range) age,
years |
47 (20-88) |
44 (18-80) |
|
Men |
55 (49%) |
45 (42%) |
|
Women |
57 (51%) |
63 (58%) |
|
Race |
||
|
African American/Black |
71 (63%) |
76 (70%) |
|
Hispanic/Latino |
16 (14%) |
14 (13%) |
|
Asian |
14 (13%) |
9 (8%) |
|
Other |
11 (10%) |
9 (8%) |
|
Clinical |
||
|
Median (10th, 90th
percentiles) |
5 (3, 10) |
4 (3, 10) |
|
duration of cough,
days |
||
|
Median (10th, 90th
percentiles) |
37·0 (36·3, 37·7) |
37·0 (36·3, 37·8) |
|
temperature, ºC |
||
|
Wheezing |
15 (13%) |
11 (10%) |
|
Fine crackles |
4 (4%) |
1 (1%) |
|
Coarse crackles |
1 (1%) |
1 (1%) |
|
Purulent nasal
discharge |
8 (7%) |
8 (7%) |
|
Exudates of tonsils or
pharynx |
0 |
0 |
|
Smoking
status |
|
|
|
Ever |
66 (59%) |
59 (55%) |
|
Current |
43 (38%) |
37 (34%) |
|
History |
|
|
|
Cocaine or heroin use |
18 (16%) |
13 (12%) |
|
Childhood asthma |
3 (3%) |
5 (5%) |
|
ACE inhibitor* use for
|
5 (5%) |
3 (3%) |
|
Diagnoses
at study entry† |
|
|
|
Upper respiratory
tract infection |
20 (18%) |
12 (11%) |
|
Rhinitis |
2 (2%) |
10 (9%) |
|
Diabetes mellitus |
5 (5%) |
3 (3%) |
|
Influenza |
1 (1%) |
3 (3%) |
|
Congestive heart
failure |
1 (1%) |
1 (1%) |
|
Gastro-oesophageal
reflux |
1 (1%) |
0 |
|
Sarcoidosis‡ |
1 (1%) |
0 |
|
Data are number of
participants unless otherwise stated. *Use of an
angiotensin-converting-enzyme inhibitor for at least 30 days. Patients
recently started on these drugs (within 30 days) were excluded before
randomisation. †All enrolled patients were diagnosed by an attending
physician as having acute bronchitis, although some had other concurrent
diagnoses. ‡Sarcoidosis, along with other chronic lung diseases, was a reason
for exclusion, but one patient with sarcoidosis was nevertheless enrolled and
randomised and therefore included in data analysis. |
||
|
Table 1: Demographic and clinical characteristics of the 220
participants at baseline |
||
On day 7, the proportion of participants
who guessed they were taking azithromycin was similar in the azithromycin and
vitamin C groups (34 of 97 [35%] vs
36 of 92 [39%], p=0·6). We unmasked treatment assignment before the end of
follow-up for two patients (both in the vitamin C group) at the request of
their treating physicians. In one of these patients, pneumonia was subsequently
diagnosed because infiltrates were seen on chest radiograph and successfully
treated on an outpatient basis with a quinolone antibiotic. The other patient
improved without any additional treatment. The azithromycin and vitamin C
groups did not differ significantly in their crude or adjusted mean
health-related quality-of-life scores on day 3 or day 7 (p>0·2; multivariate
ANCOVA with two repeated measures; table 2 and figure 2). The rate of
improvement was the same for both groups (p=0·3). There was no interaction
between baseline health-related quality-of-life score and treatment effect
(p>0·3).
|
|
Azithromycin
(n=97) |
Vitamin
C (n=92) |
Difference
vitamin C-azithromycin (95% CI)* |
p† |
|
Day 1
baseline QOL (summary score)‡ |
2·9 (1·2) |
2·6 (1·2) |
-0·3 (-0·6 to 0·03) |
0·07 |
|
Day 3
return to usual activities |
64/96 (67%) |
58/92 (63%) |
4% (-10% to 17%) |
0·6 |
|
Day 3
QOL (summary score)‡ |
1·4 (1·1) |
1·7 (1·1) |
0·3 (-0·03 to 0·55)§ |
0·08 |
|
Day 3
QOL domain |
||||
|
Activity limitations |
1·7 (1·5) |
2·1 (1·5) |
0·4 (0 to 0·8) |
0·05 |
|
Cough-related problems |
1·4 (1·1) |
1·6 (1·1) |
0·2 (-0·1 to 0·5) |
0·16 |
|
General symptoms |
1·4 (1·3) |
1·5 (1·4) |
0·1 (-0·2 to 0·5) |
0·4 |
|
Emotional functioning |
1·1 (1·2) |
1·3 (1·3) |
0·2 (-0·1 to 0·6) |
0·15 |
|
Day 7
return to usual activities† |
86/97 (89%) |
82/92 (89% ) |
0·5% (-9% to 10%) |
>0·9 |
|
Day 7
QOL summary score‡ |
0·9 (0·76) |
0·9 (0·8) |
0·03 (-0·20 to 0·26) |
0·8 |
|
Day 7
QOL domain |
||||
|
Activity limitations |
0·9 (0·9) |
1·1 (1·1) |
0·2 (-0·1 to 0·5) |
0·2 |
|
Cough-related problems |
0·9 (0·8) |
0·9 (0·9) |
0·01 (-0·2 to 0·3) |
0·9 |
|
General symptoms |
0·8 (0·9) |
0·8 (0·9) |
-0·06 (-0·3 to 0·2) |
0·6 |
|
Emotional functioning |
0·7 (1·0) |
0·8 (1·1) |
0·06 (-0·2 to 0·3) |
0·7 |
|
*Differences in
health-related quality of life on day 3 and day 7 are adjusted for baseline
scores on day 1 (ANCOVA). Differences for specific domains of quality of life
are adjusted for the domain-specific scores on day 1. A positive value for
the difference means that there was a greater improvement in the azithromycin
group. A difference of 0·5 points is taken to be the smallest important
difference. †Fisher's exact test was used to compare the proportions in each
group who had returned to usual activities on days 3 and 7. All other p
values are based on ANOVA (day 1) or ANCOVA (days 3 and 7). ‡Mean (SD). §A
difference of 0·3 points on day 3 represents a change from 2=somewhat
troubled to 1=hardly troubled at all for one in three of the symptoms or
activities addressed in the questionnaire (if the scores for the other items
remain unchanged). |
||||
|
Table 2: Baseline and follow-up results for the primary and
secondary outcomes: mean health-related quality-of-life (QOL) scores and the
proportions who had returned to usual activities on day 3 and day 7 |
||||
Figure 2: Boxplots of the baseline and follow-up results for the primary outcome: health-related quality-of-life scores Boxes extend from the
25th to the 75th percentile, the black horizontal line=median score. Whiskers
extend to the most extreme value or to 1·5 times IQR, whichever is closer.
Small open circles=outliers. |
One patient in the vitamin C group was
diagnosed with pneumonia during follow-up, compared with none in the
azithromycin group. No patient required hospital admission during the study
period.
The measurement of health-related quality
of life was internally consistent, since Cronbach's ![]() was
greater than 0·92 for each of the 3 days on which it was measured (baseline,
day 3, and day 7). The measure was also responsive to change; the average
change in score over 7 days was roughly 2 points, equivalent to more than 1·5
SDs of the baseline score.
was
greater than 0·92 for each of the 3 days on which it was measured (baseline,
day 3, and day 7). The measure was also responsive to change; the average
change in score over 7 days was roughly 2 points, equivalent to more than 1·5
SDs of the baseline score.
The effects of treatment within specific
domains of health-related quality of life approached clinical and statistical
significance on day 3 but not on day 7. On day 3, the azithromycin group was
less troubled doing daily activities and less troubled with cough and
cough-related problems than the vitamin C group (table 2), but the
between-group differences were less than 0·5, the smallest clinically important
difference. By day 7, however, differences between groups for all domains of
health-related quality of life were smaller and all CI excluded any clinically
important difference. For both the activity limitation domain and the cough
domain, there was a greater treatment effect among the most troubled patients,
although the statistical test for interaction was not significant (p>0·2).
The azithromycin and vitamin C groups did
not differ significantly in the proportion who had returned to their usual
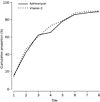
activities at work, home, or school by day 3 or by day 7 (table 2, figure 3).
By day 7, five patients in the azithromycin group and four in the vitamin C
group had made a second visit to a physician because they were not feeling
better.
Figure 3: Cumulative proportion of patients who had returned to their usual daily activities These results are a
summary of four sources of data: day 1 face-to-face interview (baseline), day
3 and day 7 telephone interviews ("Have you returned to your usual
activities at work, home, or school?" If yes: "When was that?"),
and a patient's symptom diary (returned by mail at the end of the follow-up
period). |
Among the participants contacted on day
3, 18 of 96 (19%) in the azithromycin group reported adverse effects from study
medications, compared with 19 of 92 (21%) in the vitamin C group. The most
common complaints were diarrhoea (nine vs
seven) and nausea (seven vs
three). Four participants had stopped taking the albuterol inhaler by the time
of the interview on day 3 because of perceived adverse effects, and an additional
14 had stopped because they felt sufficiently better.
On day 7, perceived adverse effects were
reported by 24 of 97 (25%) in the azithromycin group and 19 of 92 (21%) in the
vitamin C group (p>0·2). The most commonly reported adverse effects on day 7
were diarrhoea (11 vs six) and
nausea (six vs four). Only three
participants reported that they stopped the study drug because of perceived
adverse effects; all three were in the vitamin C group.
The albuterol inhaler was considered
effective by 81%, ineffective by 10%, and of uncertain value by 9%. There were
no differences between study groups. By day 7, 84 (44%) of the 189 patients
reported still using the inhaler; 11 (6%) had stopped using it because of
adverse effects, three (2%) because of perceived ineffectiveness, 64 (34%)
because they were sufficiently better, and 27 (14%) for other reasons.
Although we did an intention-to-treat
analysis of all available participants, 15 patients in the azithromycin group
and 16 in the vitamin C group were not available at follow-up. We therefore
undertook further analyses to assess the sensitivity of our results to various
assumptions about the missing data. The 31 patients lost to follow-up were more
likely than those interviewed at day 7 to have a baseline respiratory rate of
20 per min or greater (odds ratio 2·9), a baseline self-report of cocaine or
heroin use (2·7), and a lower (less troubled) baseline quality-of-life score
(0·7; each p<0·05 in a multivariate model). There were no differences by
study group. We repeated the analysis of our primary endpoint with all 220
study participants with three different methods to impute missing values: the
last value carried forward method; a best-case scenario for azithromycin; and a
worst-case scenario for azithromycin. The point estimates for the main outcome
were 0·1, 0·4, and -0·2 for the three methods. Since the three point estimates
were within 0·5 points, the smallest clinically important difference, the
study's conclusions were not considered sensitive to the method for dealing
with loss to follow-up.
![]()
|
Discussion
|
These results show that azithromycin is
no more effective than low-dose vitamin C for treatment of acute bronchitis.
Given the lack of evidence that low-dose vitamin C is beneficial, we conclude
that azithromycin is ineffective and should not be prescribed for patients with
acute bronchitis.
Our study improves on the previous
evidence in several ways. It measured the clinical outcomes most relevant to
patients: health-related quality of life and return to usual daily activities.
Our measure of disease-specific health-related quality of life was adapted from
validated instruments used for other respiratory illnesses29-31,34
and was assessed reliably with a-priori definitions of clinical significance.
Our eligibility criteria kept to a minimum diagnosis misclassification bias;5
only one of 108 patients in the control group required antibiotics for
pneumonia (apparently misdiagnosed as acute bronchitis on study entry).
Patients in both groups received intensive symptomatic treatment, including
inhaled bronchodilators.5,28 Finally, we studied a widely
prescribed, newer agent, the infrequent side-effects of which mean not only
that it allows a "best case" for antibiotic therapy to be
investigated but also that it protects concealment of study drug allocation.5
Indeed, our exit interviews confirmed that patients did not know whether or not
they had received the antibiotic. These features of our study strengthen its
internal and external validity.
The rate of improvement was more rapid
among our patients than in other studies,38-40 perhaps because the
range of illness was milder (shorter mean duration of cough at presentation and
fewer patients with abnormal chest signs) or because all patients received
aggressive symptomatic therapy with inhaled bronchodilators.5,28,
The most comprehensive meta-analysis of
antibiotics for acute bronchitis18 concluded that antibiotics
decrease cough but at the expense of drug side-effects. Although no previous
trial has tested azithromycin or measured health-related quality of life, four
other studies measured the related endpoint of return to usual activities.18
Our results for this outcome can be evaluated in the context of the other
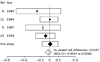
published evidence by updating the meta-analysis (figure 4). The pooled
difference of 2% favours antibiotics, but the aggregate data are compatible
with a range of values that include both benefit (up to 6%) and harm (up to 3%)
from antibiotics.
Figure 4: Meta-analysis of randomised trials that assess the difference in the proportion of patients who have returned to their usual daily activities in antibiotic and control groups For each study, the
difference in the proportion of patients who have returned to their usual
activities at follow-up (control group minus the antibiotic group) is
represented by a thin vertical line, which transects a horizontal open
rectangle depicting the 95% CI. Results less than zero favour the antibiotic
group. The solid diamonds are proportional to the studies' sample sizes. The
pooled results and 95% CI are described by the final (open) diamond. The
DerSimonian-Laird (DL) random-effects model of risk differences was used to
calculate the summary point estimate for the difference in proportions
between treatment. |
Our findings do not prove that
azithromycin provides absolutely no benefit to patients with acute bronchitis.
The 95% CI for differences between azithromycin and vitamin C on study day 3
included 0·5 for the summary quality-of-life score and for each of its
component domains. Although none of these effects persisted on day 7, our
results do not exclude the possibility that azithromycin may provide transient
benefit of little clinical significance to patients with acute bronchitis. In
addition, our sample size did not permit precise subgroup comparisons to
analyse whether azithromycin is effective for selected subgroups (for example,
elderly patients with severe symptoms). We discontinued enrolment when our
independent data-monitoring and safety committee, unaware of study group
identity, concluded that the likelihood of showing a clinically meaningful
treatment effect with a larger sample was too small to warrant continuation of
the study. Notwithstanding these reservations, our findings shift the burden of
proof to proponents of antibiotic therapy for patients with acute bronchitis.
Our study is limited in two important
ways. First, although our follow-up rate was similar in both study groups, 17%
(31 of 220) of enrolled patients did not complete the full evaluation of all
study outcomes. None of these patients was admitted to our hospital, none was
seen in the emergency department or in one of the clinics, and none died within
the month after the study period, but we cannot be certain that none had an
adverse event and either did not seek medical attention or sought attention
elsewhere. Second, our study shows that azithromycin is ineffective, but it
does not identify the best treatment for acute bronchitis. Further studies are
needed to identify more effective management strategies,41,42
because many patients with acute bronchitis require their physicians to
"do something".2,3,43 Those very reasonable demands should
be met with rigorous clinical trials, not defensive use of ineffective
antibiotics.
|
Contributors |
Arthur Evans, Shahid Husain, and Lakshmi
Durairaj designed the study, coordinated data collection, interpreted results,
and wrote the report. Laura Sadowski helped design the study, develop the
data-collection instruments, interpret results, and prepare the report. Arthur
Evans and Yue Wang analysed the data. Marjorie Charles-Damte supervised
recruitment and assessment of patients and helped design the study, develop the
data-collection instruments, interpret results, and prepare the report.
|
Conflict of interest statement |
None declared.
|
Acknowledgments |
We thank Brendan Reilly and Robert
Weinstein for their help in initiating the study, monitoring its quality and
safety, interpreting results, and writing the report; and the staff and
patients of the Ambulatory Screening Clinic, Cook County Hospital. The
Department of Medicine of Cook County Hospital funded the project from internal
funds.
![]()
|
References
|
1 Schappert SM. Ambulatory care visits to
physician offices, hospital outpatient departments, and emergency departments:
United States, 1997. Vital and Health Statistics. Series 13, No 143.
Hyattsville, MD: US Department of Health and Human Services, Centers for
Disease Control and Prevention, National Center for Health Statistics; 1999.
DHHS publication no 2000-1714.
2 Gonzales R, Bartlett JG, Besser RE, et
al. Principles of appropriate antibiotic use for treatment of uncomplicated
acute bronchitis: background. Ann
Intern Med 2001; 134: 521-29. [PubMed]
3 Gonzales R, Steiner JF, Sande MA.
Antibiotic prescribing for adults with colds, upper respiratory tract
infections, and bronchitis by ambulatory care physicians. JAMA 1997; 278: 901-04. [PubMed]
4 Huovinen P, Cars O. Control of
antimicrobial resistance: time for action. BMJ 1998; 317: 613-14. [PubMed]
5 Gonzales R, Sande M. Uncomplicated
acute bronchitis. Ann Intern Med 2001; 133: 981-91. [PubMed]
6 Stott NC, West RR. Randomized
controlled trial of antibiotics in patients with cough and purulent sputum.
BMJ 1976; 2: 556-59. [PubMed]
7 Brickfield FX, Carter WH, Johnson RE.
Erythromycin in the treatment of acute bronchitis in a community practice.
J Fam Pract 1986; 23: 119-22. [PubMed]
8 Franks P, Gleiner JA. The treatment of
acute bronchitis with trimethoprim and sulfamethoxazole. J Fam Pract 1984; 19: 185-90. [PubMed]
9 Dunlay J, Reinhardt R, Roi LD. A
placebo-controlled, double-blind trial of erythromycin in adults with acute
bronchitis. J Fam Pract 1987; 25: 137-41. [PubMed]
10 Verheij T, Hermans J, Mulder J.
Effects of doxycycline in patients with acute cough and purulent sputum: a
double-blind placebo-controlled trial. Br
J Gen Pract 1994; 44: 400-04. [PubMed]
11 Williamson HA. A randomized,
controlled trial of doxycycline in the treatment of acute bronchitis. J Fam Pract 1984; 19: 481-86. [PubMed]
12 King DE, Williams CW, Bishop L,
Schecter A. Effectiveness of erythromycin in the treatment of acute bronchitis.
J Fam Pract 1996; 42: 601-05. [PubMed]
13 Scherl ER, Riegler SL, Cooper JK.
Doxycycline in acute bronchitis: a randomized double-blind trial. J Ky Med Assoc 1987; 85: 539-41. [PubMed]
14 Hueston WJ. A comparison of albuterol
and erythromycin for the treatment of acute bronchitis. J Fam Pract 1991; 33: 476-80. [PubMed]
15 Fahey T, Stocks N, Thomas T.
Quantitative systematic review of randomized controlled trials comparing
antibiotic with placebo for acute cough in adults. BMJ 1998; 316: 906-10. [PubMed]
16 Smucny JJ, Becker LA, Glazier RH,
McIsaac. Are antibiotics effective treatment for acute bronchitis? A
meta-analysis. J Fam Pract 1998; 47: 453-60. [PubMed]
17 Bent S, Saint S, Vittinghoff E, Grady
D. Antibiotics in acute bronchitis: a meta-analysis. Am J Med 1999; 107: 62-67. [PubMed]
18 Smucny J, Fahey T, Becker L, Glazier
R, McIsaac W. Antibiotics for acute bronchitis (Cochrane Review). In: The Cochrane Library, Issue 4, 2000.
Oxford: Update Software.
19 Soepandi P, Mangunnegoro H, Yunus F,
Gunawan J. The pattern of micro-organisms and the efficacy of new macrolides in
acute lower respiratory tract infections. Respirology 1998; 3: 113-17. [PubMed]
20 Hoepelman IM, Mollers MJ, van Schie
MH, et al. A short (3-day) course of azithromycin tablets versus a 10-day
course of amoxycillin-clavulanic acid (co-amoxiclav) in the treatment of adults
with lower respiratory tract infections and effects on long-term outcome.
Int J Antimicrob Agents 1997; 9: 141-46. [PubMed]
21 Biebuyck XA. Comparison of
azithromycin and co-amoxiclav in the treatment of acute tracheobronchitis and
acute infectious exacerbations of chronic bronchitis in adults. J Int Med Res 1996; 24: 407-18. [PubMed]
22 Sternon J, Leclerq P, Knepper C, Blot
K. Azithromycin compared with clarithromycin in the treatment of adult patients
with acute purulent tracheobronchitis: a cost of illness study. J Int Med Res 1995; 23: 413-22. [PubMed]
23 Salit IE, Mederski B, Morisset R,
Trottier S. Azithromycin for the treatment of acute LRTIs: a multicenter,
open-label study. Infect Med 1998; 15: 773-77. [PubMed]
24 http://www.medscape.com/viewarticle/417417 (accessed
Feb 20, 2002).
25 Irwin RS, Boulet L-P, Cloutier MM, et
al. Managing cough as a defense mechanism and as a symptom: a consensus panel
report of the American College of Chest Physicians. Chest 1998; 114: 133S-90S. [PubMed]
26 Douglas RM, Chalker EB, Treacy B.
Vitamin C for preventing and treating the common cold (Cochrane Review). In: The Cochrane Library, Issue 4, 2000.
Oxford: Update Software.
27 Melbye H, Aasebo U, Straume B.
Symptomatic effect of inhaled fenoterol in acute bronchitis: a
placebo-controlled double-blind study. Fam
Pract 1991; 8: 216-22. [PubMed]
28 Hueston WJ. Albuterol delivered by
metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994; 39: 437-40. [PubMed]
29 Juniper EF, Guyatt GH, Ferrie PJ,
Griffith LF. Measuring quality of life in asthma. Am Rev Respir Dis 1993; 147: 832-38. [PubMed]
30 Juniper EF, Guyatt GH. Development and
testing of a new measure of health status for clinical trials in
rhinoconjunctivitis. Clin Exp Allergy 1991; 21: 77-83. [PubMed]
31 Guyatt GH, Berman LB, Townsend M,
Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in
chronic lung disease. Thorax 1987; 42: 773-78. [PubMed]
32 Guyatt GH, Nogradi S, Halcrow S, et
al. Development and testing of a new measure of health status for clinical
trials in heart failure. J Gen Intern
Med 1989; 4: 101-07. [PubMed]
33 Juniper EF, Guyatt GH, Willan A,
Griffith LE. Determining a minimal important change in disease-specific quality
of life questionnaire. J Clin
Epidemiol 1994; 47: 81-87. [PubMed]
34 Juniper EF, Guyatt GH, Griffith LE,
Perrie PJ. Interpretation of rhinoconjunctivitis quality of life questionnaire
data. J Allergy Clin Immunol 1996; 98: 843-45. [PubMed]
35 Guyatt GH, Juniper EF, Walter SD,
Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials.
BMJ 1998; 316: 690-93. [PubMed]
36 Redelmeier DA, Goldstein RS, Guyatt
GH. Assessing the minimal important difference in symptoms: a comparison of two
techniques. J Clin Epidemiol 1996; 49: 1215-19. [PubMed]
37 Miettinen OS, Nurminen M. Comparative
analysis of two rates. Stat Med 1985; 4: 213-26. [PubMed]
38 Verheij T, Hermans J, Kaptein A,
Mulder J. Acute bronchitis: course of symptoms and restrictions in patients'
daily activities. Scand J Primary
Health Care 1995; 13: 8-12. [PubMed]
39 Holmes W, Macfarlane JT, Macfarlane R,
Hubbard R. Symptoms, signs and prescribing for acute lower respiratory tract
illness. Br J Gen Pract 2001; 51: 177-81. [PubMed]
40 Macfarlane J, Holmes W, Gard P, et al.
Prospective study of the incidence, aetiology and outcome of adult lower
respiratory tract illness in the community. Thorax 2001; 56: 109-14. [PubMed]
41 Little P, Williamson I, Warner G, et
al. Open randomised trial of prescribing strategies in managing sore throat.
BMJ 1997; 314: 722-27. [PubMed]
42 Macfarlane JT, Holmes WF, Macfarlane
RM. Reducing reconsultations for acute lower respiratory tract illness with an
information leaflet: a randomised controlled study of 1006 patients in primary
care. Br J Gen Pract 1997; 47: 719-22. [PubMed]
43 Gonzales R, Sande M. What will it take
to stop physicians from prescribing antibiotics in acute bronchitis? Lancet 1995;
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.