|
|
Comparison of Early Invasive and Conservative
Strategies in Patients with Unstable Coronary Syndromes Treated with the
Glycoprotein IIb/IIIa Inhibitor Tirofiban
Christopher P. Cannon, M.D., William S. Weintraub, M.D.,
Laura A. Demopoulos, M.D., Ralph Vicari, M.D., Martin J. Frey, M.D., Nasser
Lakkis, M.D., Franz-Josef Neumann, M.D., Debbie H. Robertson, R.D., M.S., Paul
T. DeLucca, Ph.D., Peter M. DiBattiste, M.D., C. Michael Gibson, M.D., Eugene
Braunwald, M.D. for the TACTICS–Thrombolysis in Myocardial Infarction 18
Investigators
|
|
ABSTRACT
Background There is
continued debate as to whether a routine, early invasive strategy is
superior to a conservative strategy for the management of unstable
angina and myocardial infarction without ST-segment elevation.
Methods We enrolled
2220 patients with unstable angina and myocardial infarction without
ST-segment elevation who had electrocardiographic evidence of
changes in the ST segment or T wave, elevated levels of cardiac
markers, a history of coronary artery disease, or all three
findings. All patients were treated with aspirin, heparin, and the
glycoprotein IIb/IIIa inhibitor tirofiban. They were randomly
assigned to an early invasive strategy, which included routine
catheterization within 4 to 48 hours and revascularization as
appropriate, or to a more conservative (selectively invasive) strategy,
in which catheterization was performed only if the patient had
objective evidence of recurrent ischemia or an abnormal stress test.
The primary end point was a composite of death, nonfatal myocardial
infarction, and rehospitalization for an acute coronary syndrome at
six months.
Results At six
months, the rate of the primary end point was 15.9 percent with use
of the early invasive strategy and 19.4 percent with use of the
conservative strategy (odds ratio, 0.78; 95 percent confidence
interval, 0.62 to 0.97; P=0.025). The rate of death or nonfatal
myocardial infarction at six months was similarly reduced (7.3
percent vs. 9.5 percent; odds ratio, 0.74; 95 percent confidence
interval, 0.54 to 1.00; P<0.05).
Conclusions In patients
with unstable angina and myocardial infarction without ST-segment
elevation who were treated with the glycoprotein IIb/IIIa inhibitor
tirofiban, the use of an early invasive strategy significantly
reduced the incidence of major cardiac events. These data support a
policy involving broader use of the early inhibition of glycoprotein
IIb/IIIa in combination with an early invasive strategy in such
patients.
The syndrome of unstable angina and myocardial
infarction without ST-segment elevation accounts for approximately
1.4 million hospital admissions annually in the United States and 2
million to 2.5 million worldwide.1 Until
fairly recently, initial treatment focused on medical stabilization
through the use of antianginal and antithrombotic agents, including
aspirin and unfractionated or low-molecular-weight heparin.2,3,4,5 The
next step is to decide whether to refer the patient for cardiac
catheterization and revascularization, if appropriate (a routine
invasive approach), or to follow a conservative strategy, in which
cardiac procedures are performed only if the patient has spontaneous
or provoked recurrent ischemia. There is considerable debate about
which strategy is optimal, in part because the results of previous
randomized trials have been mixed.6,7,8,9,10,11
These trials were conducted before two major advances
occurred in the field: inhibitors of platelet glycoprotein IIb/IIIa
were found to reduce the risk of death, myocardial infarction, or
recurrent angina in patients with unstable angina and myocardial infarction
without ST-segment elevation, especially those who were undergoing
percutaneous coronary revascularization,12,13,14
and intracoronary stents were found to reduce the rate of
angiographically and clinically evident restenosis.15,16
Given these advances, we hypothesized that an early invasive
strategy would be superior to a more conservative approach.
Methods
Study Population
Between December 18, 1997, and December 22, 1999, a total
of 2220 patients underwent randomization. The protocol was approved
by the relevant institutional review boards, and written informed consent
was obtained from all patients. The study design has been described
previously.17
Briefly, men and women who were at least 18 years old were eligible
for inclusion if they had had an episode of angina (with an
accelerating pattern or prolonged [>20 minutes] or recurrent
episodes at rest or with minimal effort) within the preceding 24 hours,
were candidates for coronary revascularization, and had at least one
of the following: a new finding of ST-segment depression of at least
0.05 mV, transient (<20 minutes) ST-segment elevation of at least
0.1 mV, or T-wave inversion of at least 0.3 mV in at least two
leads; elevated levels of cardiac markers; or coronary disease, as
documented by a history of catheterization, revascularization, or
myocardial infarction.
Patients were excluded from the study if they met any of
the following criteria: persistent ST-segment elevation, secondary
angina,18
a history of percutaneous coronary revascularization or
coronary-artery bypass grafting within the preceding six months,
factors associated with an increased risk of bleeding,17
left bundle-branch block or paced rhythm, severe congestive heart
failure or cardiogenic shock, serious systemic disease, a serum
creatinine level of more than 2.5 mg per deciliter (221 µmol per
liter), or current participation in another study of an
investigational drug or device. Patients were also excluded if they
were taking warfarin or had received ticlopidine or clopidogrel for
more than three days before enrollment.
Medical Management
The protocol specified that patients receive 325 mg of
aspirin daily (unless contraindicated); intravenous unfractionated
heparin at an initial dose of 5000 U (as a bolus), followed by an
infusion at a rate of 1000 U per hour for 48 hours17;
and tirofiban (Aggrastat, Merck, West Point, Pa.), administered
intravenously in a loading dose of 0.4 µg per kilogram of body
weight per minute for a period of 30 minutes followed by a
maintenance infusion of 0.1 µg per kilogram per minute13
for 48 hours or until revascularization, with tirofiban administered
for at least 12 hours after percutaneous coronary revascularization
procedures. Tirofiban was available for all percutaneous coronary
revascularizations performed during follow-up. Aspirin, heparin, and
tirofiban were administered to 98 percent, more than 99 percent, and
more than 99 percent of patients, respectively. Recommended medical
therapy with beta-blockers, nitrates, and lipid-lowering agents was
administered to 82 percent, 94 percent, and 52 percent of patients,
respectively. A blood sample was obtained at base line, and levels
of troponin T (Roche Diagnostics, Indianapolis) and troponin I
(Bayer, Tarrytown, N.Y.) were analyzed later in the Thrombolysis in
Myocardial Infarction (TIMI) core laboratory. Creatine kinase and
the MB isoform of creatine kinase were measured on site every 8
hours for 24 hours at the time of randomization, for episodes of
recurrent angina suggestive of myocardial infarction, and after all
revascularization procedures. The TIMI risk score for unstable
angina and myocardial infarction without ST-segment elevation19
was determined at base line. The test evaluates patients for the
presence or absence of seven risk factors for death and ischemic
events. Patients with a score of 0, 1, or 2 are considered to be at
low risk; patients with a score of 3 or 4 are considered to be at
intermediate risk; and patients with a score of 5, 6, or 7 are
considered to be at high risk.
Treatment Strategy
Patients were randomly assigned to an early invasive
strategy or an early conservative strategy by means of a centralized
system. Patients assigned to the early invasive strategy were to
undergo coronary angiography between 4 and 48 hours after randomization
and revascularization when appropriate on the basis of coronary
anatomical findings. Patients assigned to the early conservative
strategy were treated medically and, if their condition was stable,
underwent an exercise-tolerance test (83 percent of such tests
included nuclear perfusion imaging or echocardiography performed
according to the protocol of the institution) before being
discharged. These patients were to undergo coronary angiography and
revascularization as appropriate only if they had one of the
following: prolonged or recurrent angina at rest that was associated
with electrocardiographic evidence of ischemia or changes in
cardiac-enzyme levels sufficient to meet the inclusion criteria,
hemodynamic instability, documented ischemia before the end of stage
2 of the standard Bruce protocol of a treadmill exercise test or at
any time during a pharmacologic stress test (angina accompanied by
ST-segment depression of at least 0.1 mV, ST-segment depression of
at least 0.2 mV alone, a fall in blood pressure of at least 10 mm
Hg, one large region or two smaller regions of reversible
hypoperfusion on nuclear imaging, or a new abnormality in wall
motion on stress echocardiography), unstable angina requiring
rehospitalization, Canadian Cardiovascular Society class III or IV
angina with an abnormal exercise-tolerance test, or a new myocardial
infarction. Follow-up was conducted by telephone at 30 days and 6
months, and medical records were examined to verify all end points.
A total of 27 patients (1.2 percent) had been lost to follow-up by
six months.
Statistical Analysis
The primary end point was the combined incidence of death,
nonfatal myocardial infarction, and rehospitalization for an acute
coronary syndrome at six months. End points were defined with the
use of standard TIMI definitions.20
Patients were monitored for bleeding for 24 hours after the study
medication was stopped, and major bleeding was defined as a decrease
in the blood hemoglobin level of at least 5.0 g per deciliter, the
need for the transfusion of 2 or more units of blood, the need for
corrective surgery, the occurrence of an intracranial or
retroperitoneal hemorrhage or cardiac tamponade, or any combination
of these events.12
All primary end points were adjudicated by members of an independent
clinical end-points committee who were unaware of patients' treatment
assignments.
The prospectively defined analysis of the primary end point
was a logistic-regression analysis that included terms for prior aspirin
use and an age of at least 65 years. Data on patients who were lost
to follow-up were censored at the time of the last documented
contact. We estimated that, given a 22 percent rate of the primary
end point in the conservative-strategy group, 1720 patients would be
needed to provide the study with 80 percent power to detect a
relative difference of 25 percent between the two groups. A
prespecified adjustment in the sample size to 2220 was carried out
on the basis of blinded data after 50 percent of patients had been
followed for 30 days. One interim efficacy analysis was carried out
by the data and safety monitoring board.17
Data coordination was performed by Quintiles (see the Appendix),
where the data were held for analysis. Both TIMI investigators and
Merck statisticians verified all analyses.
Results
The two groups of patients were well matched; more than 40
percent of the patients in each group were at least 65 years of age,
and one third of the patients were women (Table 1). Electrocardiographic
evidence of changes in the ST segment or T wave was present in
48 percent of the patients, and levels of troponin T were elevated
(>0.01 ng per milliliter) in 54 percent of the 1826 patients in
whom they were measured, whereas 27 percent had evidence of prior
coronary artery disease as the sole criterion for enrollment.
Base-line angiographic data in the group assigned to the early
invasive strategy revealed stenosis of the left main coronary artery
in 9 percent, three-vessel disease in 34 percent, and normal vessels
in 13 percent.
|
In the invasive-strategy group, 97 percent of the patients underwent cardiac
catheterization during the initial hospitalization a median of 22 hours
after randomization, and 60 percent underwent percutaneous coronary
revascularization or coronary-artery bypass grafting a median of 25
and 89 hours, respectively, after randomization (Table 2). In the
conservative-strategy group, 478 patients (43 percent) met the
protocol criteria for failure of medical therapy during the initial
hospitalization: 56 percent of these patients had an abnormal stress
test, 37 percent had recurrent angina at rest with
electrocardiographic changes, 4 percent had hemodynamic instability,
and 4 percent had recurrent myocardial infarction. In an additional
8 percent, medical therapy failed during follow-up, and the patients
were rehospitalized for unstable angina or myocardial infarction.
|
Of the patients who were randomly assigned to the conservative strategy,
51 percent underwent catheterization and 36 percent underwent revascularization
during the initial hospitalization. By six months the total rates of
revascularization had increased by 1 percentage point in the
invasive-strategy group and by 8 percentage points in the
conservative-strategy group. During the initial hospitalization,
coronary stents were used in 83 percent of the percutaneous coronary
revascularization procedures in the invasive-strategy group and in
86 percent of such procedures in the conservative-strategy group.
Despite being available for all percutaneous coronary
revascularization procedures conducted in both strategies, tirofiban
was used during 94 percent of procedures in the invasive-strategy
group and 59 percent of procedures in the conservative-strategy
group. The median duration of tirofiban administration was 48 and 50
hours, respectively.
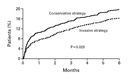
Primary End Point
The rate of primary end point — death, nonfatal myocardial
infarction, or rehospitalization for an acute coronary syndrome at
six months — was 15.9 percent with use of the early invasive
strategy and 19.4 percent with use of the conservative strategy
(odds ratio, 0.78; 95 percent confidence interval, 0.62 to 0.97;
P=0.025) (Figure
1 and Table 3).
The results of the unadjusted analysis were almost identical: the
odds ratio was 0.78 (95 percent confidence interval, 0.63 to 0.98;
P=0.028). This reduction was seen after the first week (Figure 1) and
at 30 days (P=0.009) (Table 3).
Similarly, the likelihood of death or nonfatal myocardial infarction
was significantly lower in the invasive-strategy group than in the
conservative-strategy group at 30 days (4.7 percent vs. 7.0 percent;
odds ratio, 0.65; 95 percent confidence interval, 0.45 to 0.93;
P=0.02) and at 6 months (7.3 percent vs. 9.5 percent; odds ratio,
0.74; 95 percent confidence interval, 0.54 to 1.00; P<0.05). The
benefit of the early invasive strategy was consistent among the
major subgroups, with a significantly greater benefit in the
patients with ST-segment changes at base line (P for
interaction=0.006) and in those without prior aspirin use at base
line (P for interaction=0.02) (Figure 2). The
clinical significance of the latter observation is unclear.
|
|
|
Risk Stratification
The benefit of the early invasive strategy was
significantly greater in patients with troponin T levels of more
than 0.01 ng per milliliter than in patients with levels of 0.01 ng
per milliliter or less (Table 4 and Figure 2). In
patients with a troponin T level of more than 0.01 ng per
milliliter, there was a relative reduction in the risk of the
primary end point of 39 percent with the use of the invasive
strategy rather than the conservative strategy (P<0.001), whereas
patients with a troponin T level of 0.01 ng per milliliter or less
had similar outcomes with either strategy. Similar results were
observed with the use of a troponin T cutoff point of 0.1 ng per
milliliter (Figure 2). When
patients were stratified according to the TIMI risk score,
intermediate-risk and high-risk patients derived a significant
benefit from the use of the early invasive strategy, whereas
low-risk patients had similar outcomes with the use of either
strategy (Figure
2).
|
In an attempt to determine the cause of the beneficial effect of the
early invasive strategy, we examined the outcomes among patients who
were ultimately treated with revascularization procedures and those
who received medical therapy alone (Table 5). The
benefits of the early invasive strategy were apparent at 30 days
among those who ultimately underwent revascularization, whereas
those who received medical therapy alone in each strategy group had
similar outcomes.
|
Additional Outcomes
The rates of recurrent ischemia at rest were lower in the
invasive-strategy group than in the conservative-strategy group,
both ischemia with demonstrable electrocardiographic changes (6.3
percent vs. 10.3 percent; odds ratio, 0.58; P=0.001) and ischemic
pain without electrocardiographic changes (32.3 percent vs. 49.4
percent; odds ratio, 0.49; P<0.001). Stroke occurred in 0.5 percent
of the patients in each group. The 30-day mortality rates after coronary-artery
bypass grafting and percutaneous coronary revascularization were 3.6
percent and 1.9 percent, respectively, and were similar in the two
strategy groups. Protocol-defined bleeding12
occurred in 5.5 percent of the patients in the invasive-strategy group,
as compared with 3.3 percent of those in the conservative-strategy group
(P<0.01), but the rates of major bleeding according to the
standard TIMI definition20
were not significantly different (1.9 percent vs. 1.3 percent,
P=0.24). The median length of hospitalization was one day shorter in
the invasive-strategy group than in the conservative-strategy group
(5 days vs. 6 days, P<0.001).
Discussion
This study demonstrates that among patients with unstable
angina and myocardial infarction without ST-segment elevation who
were treated with the glycoprotein IIb/IIIa inhibitor tirofiban,
an early invasive strategy was superior to a conservative strategy in
reducing the incidence of major cardiac events at 30 days and at 6
months. The benefit observed was consistent in nearly every subgroup
tested, and in a prespecified analysis that used the TIMI risk
score,19
the benefits were observed in intermediate-risk and high-risk
patients; such patients made up 75 percent of the population
studied. For the 25 percent of patients who were deemed to be at low
risk for death and ischemic events, the outcomes were similar with
the use of either strategy. We also prospectively demonstrated that
in patients with an elevated troponin T level at base line, the use
of the early invasive strategy conferred added benefit and reduced
the rates of events to those of patients who did not have an
elevated troponin T level. In addition, the 4.7 percent rate of
death or nonfatal myocardial infarction at 30 days in the
invasive-strategy group is lower than the rates reported in previous
trials of patients with unstable angina and myocardial infarction
without ST-segment elevation.12,14,21,22
Thus, we conclude that this strategy of early inhibition of
glycoprotein IIb/IIIa with tirofiban in combination with an early
invasive strategy leads to excellent outcomes and could be
considered the treatment of choice for the majority of patients with
unstable angina and myocardial infarction without ST-segment
elevation.
Two components of the early invasive strategy may explain
the benefits we observed: the early use of tirofiban and the early
timing of revascularization, the combination of which may have been
needed to achieve these benefits. In all four prior randomized trials,6,7,8,9,10,11
the rate of myocardial infarction tended to be higher in the
invasive-strategy group during the first several weeks, a finding
that is consistent with the initially increased risk of cardiac
events associated with coronary interventions. In contrast, we
observed a significantly lower rate of myocardial infarction during
this period, an effect that may be attributable to the
well-documented protection afforded by the inhibition of
glycoprotein IIb/IIIa.22
Cardiac procedures were carried out approximately two to
three days earlier in the invasive strategy than in the conservative
strategy, which appears to have averted events that would otherwise have
occurred. Indeed, the prevailing consensus little more than a decade
ago, as recommended in the 1990 guidelines of the American College
of Cardiology–American Heart Association,23
was that patients with non–Q-wave myocardial infarction should
undergo early cardiac catheterization and revascularization to avert
further events. Although prior studies failed to demonstrate the
benefit of this approach, it now appears that with the use of early
inhibition of glycoprotein IIb/IIIa and current interventional techniques,
early revascularization does prevent major cardiac events in such
patients. This further suggests that the benefit of an early
invasive strategy using inhibitors of glycoprotein IIb/IIIa should
be reevaluated in patients with myocardial infarction involving
acute ST-segment elevation.
Two types of conservative strategy were tested in earlier
studies. In the TIMI IIIB study6,7 and
the Veterans Affairs Non–Q-Wave Infarction Strategies in Hospital
(VANQWISH) trial,8
the conservative strategy involved careful monitoring for ischemia
with the use of stress testing with radionuclide or
echocardiographic imaging in nearly all patients and an
electrocardiographic criterion of the presence of ST-segment
depression of at least 0.1 mV for an abnormal test, which is
consistent with the 1994 and 2000 guidelines for the management of
unstable angina and myocardial infarction without ST-segment
elevation.1,24
This approach led to cardiac catheterization in approximately 50
percent of patients.
The conservative strategy in the Fragmin and Fast
Revascularization during Instability in Coronary Artery Disease
(FRISC) II trial used more stringent criteria for ischemia, in which
an abnormal stress test and electrocardiographic evidence of
ST-segment depression of at least 0.3 mV were required for a patient
to undergo cardiac catheterization.10
Consequently, only 10 percent of patients underwent cardiac
catheterization during the initial hospitalization. The study
reported that, as compared with this very conservative strategy, the
invasive strategy was associated with a lower rate of death or
myocardial infarction and a lower one-year mortality rate.10
Thus, because the conservative strategy they used10
was more conservative than the strategies recommended in both the
1994 and 2000 guidelines for the treatment of unstable angina and
myocardial infarction without ST-segment elevation,1,24
it was important to determine the outcomes of a more selective
invasive strategy. In addition, the antithrombotic therapy used in
FRISC II — dalteparin — has not been shown to be more efficacious
than unfractionated heparin. In our study, we used both improved
antithrombotic therapy and more sensitive monitoring for ischemia,
an approach that would also improve the outcomes of the conservative
strategy. In spite of this improved conservative strategy, we found
that the early invasive strategy, which included early inhibition of
glycoprotein IIb/IIIa and stenting, was superior in reducing the
incidence of major cardiac events.
We used immediate inhibition of glycoprotein IIb/IIIa as
part of the medical treatment of all patients. Early treatment with
tirofiban, heparin, and aspirin has been shown to reduce the incidence
of coronary thrombus,25
improve blood flow (TIMI flow grade 3),25
and lead to a reduction (by 66 percent) in the rate of death or
myocardial infarction within 48 hours as compared with treatment
with aspirin and heparin alone.12,26
Furthermore, the size of evolving myocardial infarctions without
ST-segment elevation was reduced with tirofiban (as measured on the
basis of troponin I levels)27;
this finding was confirmed in another trial that used a different
glycoprotein IIb/IIIa inhibitor and measured creatine kinase MB
levels.28,29
Finally, the reduction in the rate of death or myocardial infarction
30 days after treatment with tirofiban, heparin, and aspirin, as
compared with heparin and aspirin alone, was consistent among
patients treated medically, with percutaneous coronary
revascularization, and with coronary-artery bypass grafting.12,14,30,31
To provide patients with all these benefits, we
administered tirofiban immediately after randomization. The rate of
death or nonfatal myocardial infarction at 30 days in the
invasive-strategy group was just 4.7 percent, which compares
favorably to the rates in prior studies of patients with unstable
angina and myocardial infarction without ST-segment elevation.22
Whether similar results could be achieved in this population if
treatment with a glycoprotein IIb/IIIa inhibitor was initiated in
the catheterization laboratory only in patients undergoing percutaneous
coronary revascularization should be tested in a prospective trial.
For patients in the invasive-strategy group who underwent percutaneous
coronary revascularization after having received tirofiban, the rate
of death or myocardial infarction at 30 days was 5.3 percent (Table 5), which
also compares favorably with the results of other recent trials of
percutaneous coronary revascularization with other inhibitors of
glycoprotein IIb/IIIa.
The value of cardiac troponin levels as a means of
identifying high-risk patients has been well documented.32,33,34
Furthermore, elevations in troponin T and I levels have been found
to identify the patients who will benefit from more intensive
antithrombotic therapy, which includes low-molecular-weight heparin
and inhibition of glycoprotein IIb/IIIa.35,36,37,38 A
major objective of our study was to test prospectively the validity
of the troponin hypothesis — that the measurement of troponin T or I
at the time of presentation is useful in determining the optimal
treatment strategy. We observed that patients with elevated levels
of troponin T at base line derived a greater benefit from the early
invasive strategy than did those without elevated levels. Thus, the
use of this marker could be incorporated into management approaches
for the triage of patients with respect to an early invasive
strategy.
We found that for patients with unstable angina and
myocardial infarction without ST-segment elevation, an early
invasive strategy is superior to a conservative or a selectively
invasive strategy in reducing the incidence of major cardiac events.
This benefit applied to most patients studied, especially those at
intermediate or high risk, whereas the low-risk patients had similar
outcomes with the use of either strategy, indicating the usefulness
of early risk stratification. Our results provide evidence to
physicians of the value of broader use of a strategy of early
inhibition of glycoprotein IIb/IIIa in combination with an early
invasive approach.
Supported by Merck.
Drs. Demopoulos, DiBattiste, and DeLucca and Ms. Robertson
are employees of Merck.
* The TACTICS
(Treat Angina with Aggrastat and Determine Cost of Therapy with an
Invasive or Conservative Strategy)–Thrombolysis in Myocardial
Infarction 18 investigators are listed in the Appendix.
Source Information
From the Cardiovascular Division, Brigham and Women's
Hospital, Boston (C.P.C., E.B.); Emory University, Atlanta (W.S.W.); Merck,
West Point, Pa. (L.A.D., D.H.R., P.T.D., P.M.D.); Holmes Regional Medical
Center, Melbourne, Fla. (R.V.); the Heart Center of Sarasota, Sarasota, Fla.
(M.J.F.); Baylor College of Medicine, Houston (N.L.); Medizinische Klinik der
Technischen Universität München, Munich, Germany (F.-J.N.); and Harvard
Clinical Research Institute, Boston (C.M.G.).
Address reprint requests to Dr. Cannon at the TIMI Study
Group, Cardiovascular Division, Brigham and Women's Hospital, 75 Francis St.,
Boston, MA 02115, or at [log in to unmask].
References
- Braunwald
E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of
patients with unstable angina and non-ST-segment elevation myocardial
infarction: a report of the American College of Cardiology/American Heart
Association Task Force on Practice Guidelines (Committee on the Management
of Patients with Unstable Angina). J Am Coll Cardiol 2000;36:970-972.
[Medline]
- Théroux
P, Ouimet H, McCans J, et al. Aspirin, heparin, or both to treat acute
unstable angina. N Engl J Med 1988;319:1105-1111.
[Abstract]
- The RISC
Group. Risk of myocardial infarction and death during treatment with low
dose aspirin and intravenous heparin in men with unstable coronary artery
disease. Lancet 1990;336:827-830.
[Medline]
- Oler A,
Whooley MA, Oler J, Grady D. Adding heparin to aspirin reduces the
incidence of myocardial infarction and death in patients with unstable
angina: a meta-analysis. JAMA 1996;276:811-815.
[Medline]
- Antman
EM, Cohen M, Radley D, et al. Assessment of the treatment effect of
enoxaparin for unstable angina/non-Q-wave myocardial infarction: TIMI
11B-ESSENCE meta-analysis. Circulation 1999;100:1602-1608.
[Abstract/Full Text]
- Effects
of tissue plasminogen activator and a comparison of early invasive and
conservative strategies in unstable angina and non-Q-wave myocardial
infarction: results of the TIMI IIIB Trial. Circulation 1994;89:1545-1556.
[Abstract]
- Anderson
HV, Cannon CP, Stone PH, et al. One-year results of the Thrombolysis in
Myocardial Infarction (TIMI) IIIB clinical trial: a randomized comparison
of tissue-type plasminogen activator versus placebo and early invasive
versus early conservative strategies in unstable angina and non-Q-wave
myocardial infarction. J Am Coll Cardiol 1995;26:1643-1650. [Erratum, J Am
Coll Cardiol 2000;35:263.]
[Medline]
- Boden
WE, O'Rourke RA, Crawford MH, et al. Outcomes in patients with acute
non-Q-wave myocardial infarction randomly assigned to an invasive as
compared with a conservative management strategy. N Engl J Med
1998;338:1785-1792. [Erratum, N Engl J Med 1998;339:1091.]
[Abstract/Full Text]
- McCullough
PA, O'Neill WW, Graham M, et al. A prospective randomized trial of triage
angiography in acute coronary syndromes ineligible for thrombolytic
therapy: results of the Medicine versus Angiography in Thrombolytic
Exclusion (MATE) trial. J Am Coll Cardiol 1998;32:596-605.
[Medline]
- FRagmin
and Fast Revascularisation during InStability in Coronary artery disease
(FRISC II) Investigators. Invasive compared with non-invasive treatment in
unstable coronary-artery disease: FRISC II prospective randomised
multicentre study. Lancet 1999;354:708-715.
[Medline]
- Wallentin
L, Lagerqvist B, Husted S, Kontny F, Stahle E, Swahn E. Outcome at 1 year
after an invasive compared with a non-invasive strategy in unstable
coronary-artery disease: the FRISC II invasive randomised trial. Lancet
2000;356:9-16.
[Medline]
- The
Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited
by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators.
Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban
in unstable angina and non-Q-wave myocardial infarction. N Engl J Med
1998;338:1488-1497. [Erratum, N Engl J Med 1998;339:415.]
[Abstract/Full Text]
- The
Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study
Investigators. A comparison of aspirin plus tirofiban with aspirin plus
heparin for unstable angina. N Engl J Med 1998;338:1498-1505.
[Abstract/Full Text]
- The
PURSUIT Trial Investigators. Inhibition of platelet glycoprotein IIb/IIIa
with eptifibatide in patients with acute coronary syndromes. N Engl J Med
1998;339:436-443.
[Abstract/Full Text]
- Serruys
PW, de Jaegere P, Kiemeneij F, et al. A comparison of
balloon-expandable-stent implantation with balloon angioplasty in patients
with coronary artery disease. N Engl J Med 1994;331:489-495.
[Abstract/Full Text]
- Fischman
DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent
placement and balloon angioplasty in the treatment of coronary artery
disease. N Engl J Med 1994;331:496-501.
[Abstract/Full Text]
- Cannon
CP, Weintraub WS, Demopoulos LA, Robertson DH, Gormley GJ, Braunwald E.
Invasive versus conservative strategies in unstable angina and non-Q-wave
myocardial infarction following treatment with tirofiban: rationale and
study design of the international TACTICS-TIMI 18 Trial. Am J Cardiol
1998;82:731-736.
[Medline]
- Braunwald
E. Unstable angina: a classification. Circulation 1989;80:410-414.
[Medline]
- Antman
EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable
angina/non-ST elevation MI: a method for prognostication and therapeutic
decision making. JAMA 2000;284:835-842.
[Medline]
- Cannon
CP, McCabe CH, Wilcox RG, et al. Oral glycoprotein IIb/IIIa inhibition
with orbofiban in patients with unstable coronary syndromes (OPUS-TIMI 16)
trial. Circulation 2000;102:149-156.
[Abstract/Full Text]
- Antman
EM, McCabe CH, Gurfinkel EP, et al. Enoxaparin prevents death and cardiac
ischemic events in unstable angina/non-Q-wave myocardial infarction:
results of the Thrombolysis in Myocardial Infarction (TIMI) 11B trial.
Circulation 1999;100:1593-1601.
[Abstract/Full Text]
- Kong DF,
Califf RM, Miller DP, et al. Clinical outcomes of therapeutic agents that
block the platelet glycoprotein IIb/IIIa integrin in ischemic heart
disease. Circulation 1998;98:2829-2835.
[Abstract/Full Text]
- Gunnar
RM, Bourdillon PD, Dixon DW, et al. ACC/AHA guidelines for the early
management of patients with acute myocardial infarction: a report of the
American College of Cardiology/American Heart Association Task Force on
Assessment of Diagnostic and Therapeutic Cardiovascular Procedures
(Subcommittee to Develop Guidelines for the Early Management of Patients
with Acute Myocardial Infarction). Circulation 1990;82:664-707.
[Medline]
- Braunwald
E, Jones RH, Mark DB, et al. Diagnosing and managing unstable angina.
Circulation 1994;90:613-622.
[Abstract]
- Zhao
X-Q, Theroux P, Snapinn SM, Sax FL. Intracoronary thrombus and platelet
glycoprotein IIb/IIIa receptor blockade with tirofiban in unstable angina
or non-Q-wave myocardial infarction: angiographic results from the
PRISM-PLUS trial (Platelet Receptor Inhibition for Ischemic Syndrome Management
in Patients Limited by Unstable Signs and Symptoms). Circulation
1999;100:1609-1615.
[Abstract/Full Text]
- Boersma
E, Akkerhuis KM, Theroux P, Califf RM, Topol EJ, Simoons ML. Platelet
glycoprotein IIb/IIIa receptor inhibition in non-ST-elevation acute
coronary syndromes: early benefit during medical treatment only, with
additional protection during percutaneous coronary intervention. Circulation
1999;100:2045-2048.
[Abstract/Full Text]
- Januzzi
JL, Hahn SS, Chae CU, et al. Effects of tirofiban plus heparin versus
heparin alone on troponin I levels in patients with acute coronary
syndromes. Am J Cardiol 2000;86:713-717.
[Medline]
- Alexander
JH, Sparapani RA, Mahaffey KW, et al. Eptifibatide reduces the size and
incidence of myocardial infarction in patients with non-ST-elevation acute
coronary syndromes. J Am Coll Cardiol 1999;33:Suppl A:331A-331A.abstract
- Alexander
JH, Sparapani RA, Mahaffey KW, et al. Association between minor elevations
of creatine kinase-MB level and mortality in patients with acute coronary
syndromes without ST-segment elevation. JAMA 2000;283:347-353.
[Medline]
- Barr E,
Thornton AR, Sax FL, Snapinn SM, Theroux P. Benefit of tirofiban + heparin
therapy in unstable angina/non-Q-wave myocardial infarction patients is
observed regardless of interventional treatment strategy. Circulation
1998;98:Suppl I:I-504.abstract
- Lincoff
AM, Harrington RA, Califf RM, et al. Management of patients with acute
coronary syndromes in the United States by platelet glycoprotein IIb/IIIa
inhibition: insights from the Platelet Glycoprotein IIb/IIIa in Unstable
Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) trial.
Circulation 2000;102:1093-1100.
[Abstract/Full Text]
- Hamm CW,
Ravkilde J, Gerhardt W, et al. The prognostic value of serum troponin T in
unstable angina. N Engl J Med 1992;327:146-150.
[Abstract]
- Antman
EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels
to predict the risk of mortality in patients with acute coronary
syndromes. N Engl J Med 1996;335:1342-1349.
[Abstract/Full Text]
- Ohman EM,
Armstrong PW, Christenson RH, et al. Cardiac troponin T levels for risk
stratification in acute myocardial ischemia. N Engl J Med
1996;335:1333-1341.
[Abstract/Full Text]
- Lindhal
B, Venge P, Wallentin L. Troponin T identifies patients with unstable
coronary artery disease who benefit from long-term antithrombotic
protection. J Am Coll Cardiol 1997;29:43-48.
[Medline]
- Morrow
DA, Antman EM, Tanasijevic M, et al. Cardiac troponin I for stratification
of early outcomes and the efficacy of enoxaparin in unstable angina: a
TIMI-11B substudy. J Am Coll Cardiol 2000;36:1812-1817.
[Medline]
- Hamm CW,
Heeschen C, Goldmann B, et al. Benefit of abciximab in patients with
refractory unstable angina in relation to serum troponin T levels. N Engl J
Med 1999;340:1623-1629. [Erratum, N Engl J Med 1999;341:548.]
[Abstract/Full Text]
- Heeschen
C, Hamm CW, Goldmann B, Deu A, Langenbrink L, White HD. Troponin
concentrations for stratification of patients with acute coronary
syndromes in relation to therapeutic efficacy of tirofiban. Lancet
1999;354:1757-1762.
[Medline]
Appendix
The
following investigators and research coordinators participated in
the study (the complete list of investigators and coordinators is
available at http://www.timi.org): Steering Committee — C. Cannon
(chairman), E. Braunwald (TIMI study chairman), J. Adgey, S. Ellis,
M. Gibson, T. Henry, S. King, N. Kleiman, R. Piana, J. Popma, P.
Teirstein, W. Weintraub; Economics Committee
— W. Weintraub (chairman), S. Ellis, D. Feeny, H. Krumholtz, D.
Cohen, J. Spertus, S. Culler, A. Kosinski, E. Mahoney, C. Jurkovitz;
Clinical Events Committee — S.
Borzak (chairman), M. Attabuto, N. Bernstein, H. Cooper, R.
Giugliano, A. Jacobs, G. Koren, P. McCullough, T. Palabrica, C.
Rogers, G. Tofler; TIMI Study
Office — C.H. McCabe (project director), S. McHale; TIMI Angiographic Core Laboratory (Harvard
Clinical Research Institute, Boston) — M. Gibson, S. Marble, S.
Murphy; TIMI Serum Marker Core
Laboratory (Children's Hospital, Boston) — N. Rifai, J.
Matsubara, J. Barrow; Data and Safety
Monitoring Board — W. Parmley (chairman), E. Alderman, H.
Anderson, S. Kelsey; Data Coordinating
Center (Quintiles, Research Triangle Park, N.C.) — D.
Mackey, C. Kelly, D. Schneider, C. Tate, J. Nelson, D. Sen, J.
Davis; Merck — L. Demopoulos,
P. DiBattiste, F. Sax, G. Tarnesby, T. Bunt, D. Robertson, P.
Dellea, A. Brinton, C. Polamalu, P. DeLucca; the 25 clinical centers enrolling the most patients
(in order of enrollment) — Holmes Regional Medical
Center, Melbourne, Fla.: R. Vicari, K. Koteek; Heart Center of
Sarasota and Doctor's Hospital of Sarasota, Sarasota, Fla.: M. Frey,
N. Fichter, T. McMullen; Ben Taub General Hospital, Houston: N.
Lakkis, S. Runchey; Heart Center Research Division, Huntsville,
Ala.: R. Hunter, N. Keenum, C. Cholewa; North Mississippi Medical
Center, Tupelo: B. Bertolet, C. Bond; German Heart Center, Munich,
Germany: F. Neumann, G. Pogatsa-Murray; East Carolina School of
Medicine, Greenville, N.C.: J. Babb, D. Bembridge; University of
Oklahoma, Oklahoma City: A. Kugelmass, J. Wells; United Hospital,
St. John's Hospital, and St. Joseph's Hospital, St. Paul, Minn.:
K. Baran, C. Iacarella; Fundacion Cardio-Infantil Santafe de Bogotį,
Bogota, Colombia: M. Pineda, C. Ceballos; Cardiology of Oklahoma, Tulsa:
M. Carney, P. Flaugh; Garden City Hospital, Garden City, Mich.: W.
Back, L. Meharg, R. Morgan; Covenant Medical Center, Saginaw, Mich.:
P. Fattal, B. Garner; University of Regensburg, Regensburg, Germany:
E. Kromer, P. Schunkert, K. Kurzidim; Louisiana State University
Medical Center, Shreveport: F. Sheridan, C. Stephens; Veterans
Affairs Medical Center, Albuquerque, N.M.: S. Vernon, J. Collatz;
Centre Hospitalier des Vallees de l'Outaouais, Hull, Que., Canada:
M. Nguyen, E. Phillipe; Suburban Cardiologists, Drexel Hill, Pa.: E.
LaPorta, M. Coll; Vassar Brothers Hospital, Poughkeepsie, N.Y.: D.
O'Dea, P. Soriano; San Diego Veterans Affairs Medical Center, San
Diego, Calif.: W. Penny, G. Poteat; University of Michigan Medical
Center, Ann Arbor: E. Bates, J. Fortino; Medical Clinic I,
University of Aachen, Aachen, Germany: P. Hanrath, K.-C. Koch;
Montefiore Medical Center, Bronx, N.Y.: H. Mueller, J. Kouns, J.
Cosico; Grass Valley Cardiology, Grass Valley, Calif.: P. Callaham,
R. Schnabel-Petersen; Harborview Medical Center and University of
Washington Medical Center, Seattle: M. Corson, C. Brown, R. Divine;
Akron General Medical Center, Akron, Ohio: J. Hodsden, D. Hudock.
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.