|
|
Measurement of C-Reactive Protein for the
Targeting of Statin Therapy in the Primary Prevention of Acute Coronary Events
Paul M. Ridker, M.D., M.P.H., Nader
Rifai, Ph.D., Michael Clearfield, D.O., John R. Downs, M.D., Stephen E. Weis,
D.O., J. Shawn Miles, M.D., Antonio M. Gotto, Jr., M.D., D.Phil. for the Air
Force/Texas Coronary Atherosclerosis Prevention Study Investigators ABSTRACT
Background Elevated
levels of C-reactive protein, even in the absence of hyperlipidemia,
are associated with an increased risk of coronary events. Statin
therapy reduces the level of C-reactive protein independently of its
effect on lipid levels. We hypothesized that statins might prevent
coronary events in persons with elevated C-reactive protein levels
who did not have overt hyperlipidemia.
Methods The level of
C-reactive protein was measured at base line and after one year in
5742 participants in a five-year randomized trial of lovastatin for
the primary prevention of acute coronary events.
Results The rates of
coronary events increased significantly with increases in the
base-line levels of C-reactive protein. Lovastatin therapy reduced
the C-reactive protein level by 14.8 percent (P<0.001), an effect
not explained by lovastatin-induced changes in the lipid profile. As
expected, lovastatin was effective in preventing coronary events in
participants whose base-line ratio of total cholesterol to
high-density lipoprotein (HDL) cholesterol was higher than the
median ratio, regardless of the level of C-reactive protein (number
needed to treat for five years to prevent 1 event, 47; P=0.005).
However, lovastatin was also effective among those with a ratio of
total to HDL cholesterol that was lower than the median and a C-reactive
protein level higher than the median (number needed to treat, 43;
P=0.02). In contrast, lovastatin was ineffective among participants with
a ratio of total to HDL cholesterol and a C-reactive protein level
that were both lower than the median (number needed to treat, 983;
P=0.87).
Conclusions Statin
therapy may be effective in the primary prevention of coronary
events among persons with relatively low lipid levels but with
elevated levels of C-reactive protein.
Both the Air Force/Texas Coronary Atherosclerosis
Prevention Study (AFCAPS/TexCAPS) and the West of Scotland Coronary
Prevention Study demonstrated that inhibitors of
hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase (statins)
reduce the risk of first coronary events.1,2
However, the use of statins for primary prevention has not been
widely adopted, in part because the number of persons who need to be
treated to prevent one clinical event is relatively large and the
cost of this approach is substantial.3
A method of distinguishing high-risk from low-risk patients might
make possible better targeting of statin therapy for primary prevention.4 For
example, restricting statin use to those with overt hyperlipidemia
improves the cost effectiveness of the therapy,5 and
the current guidelines of the National Cholesterol Education Program
recommend that statins be prescribed for primary prevention when
low-density lipoprotein (LDL) cholesterol levels exceed 160 mg per
deciliter (4.14 mmol per liter).6
Unfortunately, half of all coronary events occur in persons without
overt hyperlipidemia.7
Thus, lipid screening alone may fail to identify all high-risk subgroups
that are likely to benefit from statin therapy.
Several studies suggest that measurement of the inflammatory marker
C-reactive protein may provide a useful method of assessing the risk
of cardiovascular disease in apparently healthy persons, particularly
when lipid levels are low.8,9,10,11,12
Furthermore, statin therapy has been shown to reduce C-reactive
protein levels independently of its effect on cholesterol,13,14
and statins may have antiinflammatory properties.15
Although the addition of an evaluation of C-reactive protein levels
to standard lipid screening has been shown to improve risk
prediction in the primary prevention of acute coronary events,9,16
there are no data demonstrating that C-reactive protein screening
can identify subgroups of patients who are more or less likely to
benefit from statin therapy.
To address this issue, we measured the level of C-reactive protein
both at base line and after one year of follow-up among 5742 of
the 6605 participants enrolled in a randomized, double-blind, placebo-controlled
trial of lovastatin in the primary prevention of acute coronary
events in persons with average levels of total cholesterol and
below-average levels of high-density lipoprotein (HDL) cholesterol.1
Methods
AFCAPS/TexCAPS was a primary-prevention trial of lovastatin, conducted
between 1990 and 1998, that included 6605 men and women at two sites
in Texas, the Lackland Air Force Base and the University of North
Texas Health Science Center.1,17
Men 45 to 73 years old and postmenopausal women 55 to 73 years old
who had average levels of total and LDL cholesterol and below-average
levels of HDL cholesterol were eligible. Persons with uncontrolled hypertension,
secondary hyperlipidemia, diabetes requiring insulin, or a body mass
50 percent greater than desirable were excluded.
Participants who provided written informed consent, met all the
entrance criteria, and completed a 12-week run-in period during
which they followed the American Heart Association Step I diet were
randomly assigned to receive either lovastatin (20 mg per day) or
matching placebo. The dose of lovastatin was increased in a
double-blind manner to 40 mg of lovastatin per day if the LDL
cholesterol level was higher than 110 mg per deciliter (2.84 mmol
per liter) at the three-month visit. We conducted follow-up for an
average of 5.2 years to monitor the occurrence of first acute
coronary events, which were prospectively defined as fatal or
nonfatal myocardial infarction, unstable angina, or sudden death
from cardiac causes. As we previously reported,1
assignment to the lovastatin group was associated with a rate of
reaching this primary clinical end point that was 37 percent lower
than that in the placebo group (relative risk, 0.63; 95 percent
confidence interval, 0.50 to 0.79; P<0.001).
Laboratory Analyses
A highly sensitive latex-based immunoassay (Dade Behring, Newark,
Del.) was used to determine the levels of C-reactive protein in
blood obtained at the time of randomization and at one year.18
Lipid levels were measured in a laboratory accredited by the Lipid
Standardization Program of the Centers for Disease Control and
Prevention. In total, 5742 of the 6605 participants (87 percent) had
blood available for analysis and underwent successful evaluation for
high-sensitivity C-reactive protein and lipid levels. The median LDL
cholesterol level (149.1 mg per deciliter [3.86 mmol per liter]) and
the median ratio of total to HDL cholesterol (5.96) among these 5742
participants were virtually identical to the median level and ratio
(149.3 mg per deciliter [3.86 mmol per liter] and 5.98,
respectively) in the study cohort as a whole.
Statistical Analysis
After the study cohort had been divided into quartiles on the
basis of C-reactive protein levels, Cox regression analysis was
used to test for an association between base-line levels of
C-reactive protein and the risk of acute coronary events. Adjusted
risk estimates were obtained from analyses that also controlled for
age, sex, smoking status, hypertension, parental history with
respect to coronary disease, and lipid levels.19
Spearman correlation coefficients were used to evaluate potential
relations between C-reactive protein levels and lipid levels at
study entry and between the change in C-reactive protein levels and
the change in lipid values by the end of one year of therapy. The
percentage change in C-reactive protein levels that was associated
with the use of lovastatin was also computed and compared with the percentage
change in C-reactive protein levels among those assigned to the
placebo group.
To evaluate the efficacy of lovastatin as compared with placebo
in subgroups defined according to base-line levels of lipids and
C-reactive protein, we divided the study cohort into four groups of
approximately equal size: those with an LDL cholesterol level lower
than the median (less than 149.1 mg per deciliter) and a C-reactive
protein level lower than the median (less than 0.16 mg per
deciliter) (1448 participants); those with an LDL cholesterol level
lower than the median and a C-reactive protein level higher than the
median (1428 participants); those with an LDL cholesterol level
higher than the median and a C-reactive protein level lower than the
median (1420 participants); and those with an LDL cholesterol level
higher than the median and a C-reactive protein level higher than
the median (1446 participants). We then computed the reductions in
relative risk associated with lovastatin as compared with placebo in
each of these four groups, as well as the number of persons who
would have to be treated for five years to prevent one acute
coronary event.
To determine whether any observed effects within these groups
were sensitive to the choice of lipid variable and to address the
fact that the AFCAPS/TexCAPS trial enrolled participants with
below-average HDL cholesterol levels, we repeated these analyses
using the median base-line ratio of total to HDL cholesterol (5.96)
rather than the median base-line LDL cholesterol level.
Results
The overall distribution of C-reactive protein values in this
study was similar to that reported in previous studies of primary prevention.8,9,10,11
The mean and median levels of C-reactive protein were 0.31 and 0.16
mg per deciliter, respectively, and the ranges of C-reactive protein
levels in the four quartiles were less than 0.08 mg per deciliter,
0.08 to less than 0.16 mg per deciliter, 0.16 to 0.35 mg per deciliter,
and greater than 0.35 mg per deciliter.
Our data provided minimal evidence of an association between base-line
C-reactive protein levels and base-line lipid levels; the Spearman
correlation coefficients for the relations between C-reactive protein
levels and total, LDL, and HDL cholesterol and triglyceride levels
and the ratio of total to HDL cholesterol were 0.069, 0.012, –0.058,
0.129, and 0.092, respectively. Thus, less than 2 percent of the
variance in base-line C-reactive protein levels was determined by
lipid factors.
Overall, the rates of coronary events increased with the base-line
levels of C-reactive protein, so that the relative risks of coronary
events in participants assigned to the placebo group as compared
with those in the lovastatin group were 1.0, 1.2, 1.3, and 1.7 for
the lowest to highest quartile of base-line levels of C-reactive
protein (P=0.01). In unadjusted analyses, the risk of acute coronary
events increased by 21 percent with each increasing quartile of
base-line C-reactive protein levels (95 percent confidence interval,
4 to 41 percent). In similar analyses with control for age, sex,
smoking status, hypertension, parental history with respect to
coronary disease, and lipid levels, the increase in risk associated
with a one-quartile increase in the C-reactive protein level (17
percent; 95 percent confidence interval, 3 to 33 percent) was almost
identical in magnitude to that associated with an increase of 1.0 in
the ratio of total to HDL cholesterol (18 percent; 95 percent
confidence interval, 5 to 33 percent).
Lovastatin therapy was associated with a statistically significant
14.8 percent reduction in the median level of C-reactive protein (95
percent confidence interval, 12.5 to 17.4 percent; P<0.001) at
the end of the first year of treatment (Table 1). By
contrast, assignment to the placebo group had no effect on the
median level of C-reactive protein (median percentage change, 0.0;
95 percent confidence interval, 0.0 to 5.3 percent), although there
were more participants with an increase in C-reactive protein levels
than with a decrease. Thus, the difference between the lovastatin
group and the placebo group in terms of the change in C-reactive protein
levels over time was significant (P<0.001). This effect of
lovastatin on the level of C-reactive protein was not related to the
effect of lovastatin on lipid levels; among the participants in the
lovastatin group, the Spearman correlation coefficients for the
relation between the percentage change in C-reactive protein level
and the percentage change in total, LDL, and HDL cholesterol and
triglyceride levels and the ratio of total to HDL cholesterol were
–0.001, 0.014, –0.079, –0.013, and 0.061, respectively. Thus,
virtually none of the observed variance in the effect of lovastatin
on C-reactive protein levels could be explained by
lovastatin-induced changes in lipid fractions.
|
Table 2
presents the results of efficacy analyses for lovastatin in
subgroups of participants delineated according to LDL cholesterol and
C-reactive protein levels. As expected, given the overall findings
of the trial, lovastatin was clinically effective among participants
with LDL cholesterol levels higher than the median, regardless of
their C-reactive protein levels (relative risk of acute coronary
events, 0.53; 95 percent confidence interval, 0.37 to 0.77; number
needed to treat, 42; P=0.001). However, lovastatin was also
clinically effective among those with LDL cholesterol levels lower
than the median and C-reactive protein levels higher than the median
(relative risk, 0.58; 95 percent confidence interval, 0.34 to 0.98;
number needed to treat, 48; P=0.04). In contrast, among the
participants with LDL cholesterol and C-reactive protein levels that
were both lower than the median, the point estimate did not indicate
that lovastatin reduced the risk of acute coronary events (relative
risk, 1.08; 95 percent confidence interval, 0.56 to 2.08; P=0.74).
In these analyses, formal testing for a multiplicative interaction
among lovastatin, lipids, and C-reactive protein indicated
borderline statistical significance (P=0.06).
|
We evaluated the robustness of these analyses by stratifying the
study cohort on the basis of the median base-line ratio of total to
HDL cholesterol, rather than on the basis of the base-line LDL
cholesterol level, and the results were nearly identical (Table 3).
Specifically, lovastatin was highly effective among participants
with a base-line ratio of total to HDL cholesterol that was higher
than the median (number needed to treat, 47; P=0.005). However,
lovastatin was also highly effective among those with a ratio of
total to HDL cholesterol lower than the median and a C-reactive
protein level higher than the median (number needed to treat, 43;
P=0.02). In contrast, lovastatin was far less effective among those
with a ratio of total to HDL cholesterol lower than the median who
also had a C-reactive protein level lower than the median (number
needed to treat, 983; P=0.87) (Table 3).
|
The rates of events among the participants in the placebo group who
had lipid levels lower than the median and C-reactive protein levels
higher than the median were just as high as the rates of events
among those with overt hyperlipidemia (Table 2 and
Table 3).
Moreover, lovastatin was clinically effective in reducing the risk
of acute coronary events among participants with lipid levels lower
than the median and C-reactive protein levels higher than the
median, but not among those with lipid levels and C-reactive protein
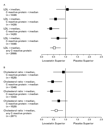
levels that were both lower than the median (Figure 1).
|
In these data, the observed efficacy of lovastatin in preventing acute
coronary events was not statistically significant among the
participants with lipid levels and C-reactive protein levels that
were both higher than the median (Figure 1).
However, in each of the two subgroups defined according to these
criteria, the point estimates of effect indicate an overall net
benefit with lovastatin. Furthermore, there was no evidence of any
statistically significant difference between the efficacy of
lovastatin among the participants with lipid levels and C-reactive
protein levels that were both higher than the median and its
efficacy among those with lipid levels higher than the median but
C-reactive protein levels lower than the median; these data suggest
that any small differences between the results in these subgroups
probably represent the effects of chance. Finally, because the rates
of events were high among participants with lipid levels and
C-reactive protein levels that were both higher than the median, the
number needed to treat in these subgroups was well below the number
considered the threshold for justifying treatment for primary
prevention. Indeed, the number needed to treat among participants
with lipid levels and C-reactive protein levels that were both
higher than the median was of similar magnitude to that found in
subgroups in which the efficacy of lovastatin was clearly
statistically significant (Table 2 and Table 3).
Discussion
Among the participants in AFCAPS/TexCAPS, base-line C-reactive
protein levels were an independent predictor of first acute coronary
events. Furthermore, lovastatin appeared to be highly effective in
reducing the risk of acute coronary events in participants with
elevated C-reactive protein levels but no hyperlipidemia. Indeed,
among participants with either an LDL cholesterol level or a ratio
of total to HDL cholesterol that was lower than the median but a
C-reactive protein level higher than the median, the number needed
to treat with lovastatin to prevent one clinical event was virtually
identical to that among participants with lipid levels higher than
the median. These analyses thus raise the possibility that statin
therapy may be clinically effective in persons without
hyperlipidemia and suggest that evaluation of the C-reactive protein
level may provide a method for the appropriate targeting of statin
therapy for primary prevention.20
Finally, lovastatin significantly reduced C-reactive protein levels
independently of its effect on lipids.
The results of this study have several implications. First, the
current data confirm in a large population of apparently healthy men
and women that C-reactive protein can be used to determine the risk
of acute coronary events. The effect of the C-reactive protein level
on risk was independent of all other factors, including lipid
levels, known to predict clinical coronary outcomes. Thus, as in our
earlier studies,8,9,16
the current data are consistent with the hypothesis that the
addition of an evaluation of the C-reactive protein level to the
standard lipid evaluation may provide an improved method of
identifying persons at high risk.
Second, in this double-blind trial, the use of lovastatin resulted
in a 14.8 percent reduction in median C-reactive protein levels after
one year (P<0.001), whereas no change in C-reactive protein
levels occurred in participants in the placebo group. Thus, the
current data also confirm the findings of the Cholesterol and
Recurrent Events (CARE) trial, in which assignment to pravastatin therapy
led to a 17.4 percent reduction in median C-reactive protein levels
over a five-year period.13
As in the CARE trial, the effect of lovastatin on C-reactive protein
levels in our study appeared to be unrelated to any effect of
HMG-CoA reductase inhibition on plasma lipid levels. Together, these
clinical data provide evidence of nonlipid effects of this class of
agents13,14,15
and suggest that statins may lead to the stabilization of plaque in
part through antiinflammatory mechanisms.21,22,23
Third, although our study is hypothesis-generating, the fact that
lovastatin was highly effective among participants without marked
hyperlipidemia but with elevated levels of C-reactive protein may
have implications for the use of HMG-CoA reductase inhibitors in
primary prevention. As outlined in the current guidelines of the
National Cholesterol Education Program, strategies to target statin
therapy in primary prevention rely largely on LDL cholesterol
screening, an approach that results in a reduction in the number
needed to treat to prevent one event and improves the cost
effectiveness of these agents.5,6
However, as the current data suggest, lovastatin may be highly
effective among persons with average and below-average LDL
cholesterol levels who have C-reactive protein levels higher than
the median. Thus, if the number needed to treat is used to estimate
the effect of therapy in primary prevention, then C-reactive protein
screening might provide an additional method for targeting the use
of statins, particularly when lipid levels are normal or low.
In the current study, the magnitude of the increase in risk associated
with higher levels of C-reactive protein is somewhat smaller than
that observed in previous studies.8,9,10,11
Several aspects of the design of our study probably account for this
difference. For example, obese persons and diabetic patients requiring
insulin were excluded from the study. Since these groups have
elevated C-reactive protein levels and are at increased risk for
cardiovascular disease,24
their exclusion would tend to lead to underestimation of the
predictive value of the C-reactive protein level. Similarly, because
C-reactive protein and lipid levels appear additive in their ability
to predict the risk of cardiovascular disease,9,16
the further exclusion from the study of persons with severe
hyperlipidemia would also tend to reduce the predictive value of the
C-reactive protein level. Finally, nearly 20 percent of the
participants in AFCAPS/TexCAPS were taking aspirin, a drug that has
also been shown to reduce the effect of C-reactive protein on
vascular risk.8
For all of these reasons, estimates of the risk associated with
C-reactive protein derived from data from our study cohort would be
expected to be lower than those found in unselected populations.20
These issues would not, however, affect the validity of observations
made in the context of this study with regard to statin therapy and
C-reactive protein, since the participants were assigned to
treatment groups in a double-blind manner, without knowledge of
C-reactive protein values.
From a clinical perspective, it is important to recognize that
half of all heart attacks occur among persons without overt hyperlipidemia7 and
thus that novel approaches to the determination of the risk of
cardiovascular disease as well as to intervention are needed to
improve resource allocation in the primary prevention of myocardial
infarction.25
In a recent study of patients with a history of myocardial
infarction, randomized use of statin therapy reduced the risk of
recurrent coronary events associated with elevated levels of
C-reactive protein.26
In the current study of primary prevention, statin therapy was found
to reduce the risk of acute coronary events associated with
C-reactive protein, even in the absence of hyperlipidemia. Thus,
these hypothesis-generating clinical studies, together with the
recognition that, biologically, atherosclerosis is in part an
inflammatory disease21
and that the lowering of lipid levels may represent an
antiinflammatory process,22
appear to provide a rationale for considering wider use of statins
than is typically achieved in current practice. Nonetheless, despite
large differences in the number needed to treat in this study, the
absolute number of events that occurred in each of the four
subgroups of participants was small, and formal testing for a
multiplicative interaction among lovastatin, lipids, and C-reactive
protein indicated borderline statistical significance (P=0.06).
Thus, randomized trials of statin therapy among persons without
overt hyperlipidemia but with evidence of systemic inflammation are
needed in order to test these hypotheses directly.
Supported by grants from the National Heart, Lung, and Blood Institute
(HL58755) and the Leducq Foundation, Paris. Dr. Ridker is also the
recipient of an Established Investigator Award from the American
Heart Association and a Doris Duke Distinguished Clinical Scientist
Award from the Doris Duke Charitable Foundation. The AFCAPS/TexCAPS
trial was supported by grants from Merck.
Dr. Ridker is named as a coinventor on patent applications filed
for the use of inflammatory markers in coronary artery disease. Drs.
Gotto, Clearfield, Downs, and Weis have either served as consultants
to Merck (the manufacturer of lovastatin) or received honorariums
from Merck.
We are indebted to Ms. JoAnne Emerson and Mr. Thomas Cook for
their assistance with this project.
Source Information
From the Center for Cardiovascular Disease Prevention, Brigham and
Women's Hospital and Harvard Medical School, Boston (P.M.R., N.R., J.S.M.); the
University of North Texas Health Science Center, Fort Worth (M.C., S.E.W.);
Wilford Hall Medical Center, Lackland Air Force Base, San Antonio, Tex.
(J.R.D.); and Weill Medical College of Cornell University, New York (A.M.G.).
Address reprint requests to Dr. Ridker at the Center for
Cardiovascular Disease Prevention, Brigham and Women's Hospital, 900
Commonwealth Ave. E., Boston, MA 02215, or at [log in to unmask].
References
- Downs
JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary
events with lovastatin in men and women with average cholesterol levels:
results of AFCAPS/TexCAPS. JAMA 1998;279:1615-1622.
[Medline]
- Shepherd
J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with
pravastatin in men with hypercholesterolemia. N Engl J Med
1995;333:1301-1307.
[Abstract/Full Text]
- Goldman
L, Weinstein MC, Goldman PA, Williams LW. Cost-effectiveness of HMG-CoA
reductase inhibition for primary and secondary prevention of coronary
heart disease. JAMA 1991;265:1145-1151.
[Medline]
- Jacobson
TA, Schein JR, Williamson A, Ballantyne CM. Maximizing the
cost-effectiveness of lipid-lowering therapy. Arch Intern Med
1998;158:1977-1989.
[Medline]
- Garber
AM. Using cost-effectiveness analysis to target cholesterol reduction. Ann
Intern Med 2000;132:833-835.
[Medline]
- Expert
Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in
Adults. Summary of the second report of the National Cholesterol Education
(NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood
Cholesterol in Adults (Adult Treatment Panel II). JAMA 1993;269:3015-3023.
[Medline]
- Braunwald
E. Shattuck Lecture -- cardiovascular medicine at the turn of the
millennium: triumphs, concerns, and opportunities. N Engl J Med
1997;337:1360-1369.
[Full Text]
- Ridker
PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin,
and the risk of cardiovascular disease in apparently healthy men. N Engl J
Med 1997;336:973-979. [Erratum, N Engl J Med 1997;337:356.]
[Abstract/Full Text]
- Ridker
PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers
of inflammation in the prediction of cardiovascular disease in women. N
Engl J Med 2000;342:836-843.
[Abstract/Full Text]
- Koenig
W, Sund M, Frohlich M, et al. C-reactive protein, a sensitive marker of
inflammation, predicts future risk of coronary heart disease in initially
healthy middle-aged men: results from the MONICA (Monitoring Trends and
Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to
1992. Circulation 1999;99:237-242.
[Abstract/Full Text]
- Danesh
J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart
disease: prospective study and updated meta-analyses. BMJ 2000;321:199-204.
[Abstract/Full Text]
- Ridker
PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis:
a comparison of C-reactive protein, fibrinogen, homocysteine,
lipoprotein(a), and standard cholesterol screening as predictors of
peripheral arterial disease. JAMA 2001;285:2481-2485.
[Medline]
- Ridker
PM, Rifai N, Pfeffer M, Sacks F, Braunwald E. Long-term effects of
pravastatin on plasma concentration of C-reactive protein. Circulation
1999;100:230-235.
[Abstract/Full Text]
- Ridker
PM, Rifai N, Lowenthal SP. Rapid reduction in C-reactive protein with
cerivastatin among 785 patients with primary hypercholesterolemia.
Circulation 2001;103:1191-1193.
[Abstract/Full Text]
- Farmer
JA. Pleiotropic effects of statins. Curr Atheroscler Rep 2000;2:208-217.
[Medline]
- Ridker
PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive
value of total and HDL cholesterol in determining risk of first myocardial
infarction. Circulation 1998;97:2007-2011.
[Abstract/Full Text]
- Downs
JR, Beere PA, Whitney E, et al. Design and rationale of the Air
Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am
J Cardiol 1997;80:287-293.
[Medline]
- Rifai N,
Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity
C-reactive protein assay. Clin Chem 1999;45:2136-2141.
[Abstract/Full Text]
- Gotto
AM, Whitney E, Stein EA, et al. Relation between baseline and on-treatment
lipid parameters and first acute major coronary events in the Air
Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS).
Circulation 2000;101:477-484.
[Abstract/Full Text]
- Ridker
PM. High-sensitivity C-reactive protein: potential adjunct for global risk
assessment in the primary prevention of cardiovascular disease.
Circulation 2001;103:1813-1818.
[Abstract/Full Text]
- Ross R.
Atherosclerosis -- an inflammatory disease. N Engl J Med 1999;340:115-126.
[Full Text]
- Libby P.
Molecular bases of the acute coronary syndromes. Circulation 1995;91:2844-2850.
[Full Text]
- Ferro D,
Parrotto S, Basili S, Alessandri C, Violi F. Simvastatin inhibits the
monocyte expression of proinflammatory cytokines in patients with
hypercholesterolemia. J Am Coll Cardiol 2000;36:427-431.
[Medline]
- Visser
M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive
protein levels in overweight and obese adults. JAMA 1999;282:2131-2135.
[Medline]
- Ridker
PM. Evaluating novel cardiovascular risk factors: can we better predict
heart attacks? Ann Intern Med 1999;130:933-937.
[Medline]
- Ridker
PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of
coronary events after myocardial infarction in patients with average
cholesterol levels. Circulation 1998;98:839-844.
[Abstract/Full Text]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.