|
|
The Irritable Bowel Syndrome
Brenda J. Horwitz, M.D. and Robert S.
Fisher, M.D. In
1849, Cumming1
said of the irritable bowel syndrome, "The bowels are at one
time constipated, another lax, in the same person. How the disease
has two such different symptoms I do not profess to explain."
Over the years, the unexplained gastrointestinal symptoms of the
irritable bowel syndrome have been described in various terms,
including mucous colitis, spastic colitis, nervous colon, and
irritable colon. The irritable bowel syndrome and nonulcer dyspepsia
are the most common functional gastrointestinal disorders.
The irritable bowel syndrome is defined on the basis of the recently
modified Rome criteria as the presence for at least 12 weeks (not
necessarily consecutive) in the preceding 12 months of abdominal
discomfort or pain that cannot be explained by structural or
biochemical abnormalities and that has at least two of the following
three features: pain is relieved with defecation, its onset is
associated with a change in the frequency of bowel movements
(diarrhea or constipation), or its onset is associated with a change
in the form of the stool (loose, watery, or pellet-like).2
The syndrome can be divided into four subcategories according to
whether the predominant symptom is abdominal pain, diarrhea, constipation,
or constipation alternating with diarrhea.
Epidemiologic Features
Approximately 15 percent of U.S. adults report symptoms that are
consistent with the diagnosis of the irritable bowel syndrome3;
the disease affects three times as many women as men. Whether this
difference reflects a true predominance of the disorder among women
or merely the fact that women are more likely to seek medical care
has not been determined. The irritable bowel syndrome is the most
common diagnosis made by gastroenterologists in the United States4 and
accounts for 12 percent of visits to primary care providers.5 It
is estimated that only 25 percent of persons with this condition
seek medical care for it, and studies suggest that those who seek
care are more likely to have behavioral and psychiatric problems
than are those who do not seek care.6 In
addition, patients with a diagnosis of the irritable bowel syndrome
are at increased risk for other, nongastrointestinal functional
disorders such as fibromyalgia and interstitial cystitis.7,8 The
irritable bowel syndrome accounts for an estimated $8 billion in
direct medical costs and $25 billion in indirect costs annually in
the United States.9
Pathophysiologic Features
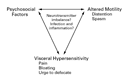
Altered bowel motility, visceral hypersensitivity, psychosocial
factors, an imbalance in neurotransmitters, and infection have all
been proposed as playing a part in the development of the irritable
bowel syndrome (Figure
1).
|
Altered Bowel Motility
Over the past 50 years, alterations in the contractility of the
colon and small bowel have been described in patients with the
irritable bowel syndrome. Psychological or physical stress10
and ingestion of food11
may alter the contractility of the colon. Abnormal motility of the
small intestine during fasting, such as loss of the migrating motor
complex12
and the presence of both discrete, clustered contractions and
prolonged, propagated contractions,13
has been described in patients with the irritable bowel syndrome. In
addition, an exaggerated contractile response to a high-fat meal has
been reported.13
Pain is more frequently associated with irregular motor activity of
the small intestine in patients with this syndrome than in normal
controls or patients with inflammatory bowel disease.12
Visceral Hypersensitivity
Balloon-distention studies of the rectosigmoid14
and the ileum15
have shown that patients with the irritable bowel syndrome experience
pain and bloating at balloon volumes and pressures that are significantly
lower than those that induce pain in control subjects, a phenomenon
referred to as visceral hypersensitivity. One possible explanation
is that the sensitivity of receptors in the viscus is altered
through the recruitment of silent nociceptors in response to
ischemia, distention, intraluminal contents, infection, or
psychiatric factors.
There may be increased excitability of the neurons in the dorsal
horn of the spinal cord, an area rich in neurotransmitters such as
catecholamines and serotonin. Centrally, there may be differences in
the way the brain modulates afferent signals from the dorsal-horn neurons
through the ascending pathways. Functional magnetic resonance
imaging16,17
and positron-emission tomography18,19
of the brain show different levels of activation in the thalamus and
the anterior cingulate cortex after balloon distention of the rectum
in patients with the irritable bowel syndrome, as compared with
normal subjects.
These findings, although controversial, suggest a primary central
defect of visceral pain processing. Some authors have suggested that
hypervigilance rather than true visceral hypersensitivity may be
responsible for the low pain threshold in patients with the
irritable bowel syndrome.
Psychosocial Factors
Psychological stress can alter motor function in the small bowel11
and colon,20
both in normal subjects and in patients with the irritable bowel
syndrome. Up to 60 percent of patients seen at referral centers have
psychiatric symptoms such as somatization, depression, and anxiety,
and patients with a diagnosis of the irritable bowel syndrome are
more likely to have these symptoms than are persons who have never
sought medical care for bowel problems.21,22
When present, psychiatric disturbances influence overall use of
health care services and the ability to cope with symptoms. The
presence or absence of a history of abuse in childhood (sexual,
physical, or both) is correlated with the severity of symptoms in
patients with the irritable bowel syndrome.23
It has even been proposed that experiences early in life may affect
the central nervous system and confer a predisposition to a state of
hypervigilance.
Neurotransmitter Imbalance
Recent studies have suggested that neurotransmitters are involved
in the pathogenesis of the irritable bowel syndrome. Five percent of
serotonin is located in the central nervous system, and the remaining
95 percent is in the gastrointestinal tract, within enterochromaffin
cells, neurons, mast cells, and smooth-muscle cells. When released
by enterochromaffin cells, serotonin stimulates extrinsic vagal
afferent nerve fibers and intrinsic enteric afferent nerve fibers,
resulting in such physiological responses as intestinal secretion
and the peristaltic reflex and in such symptoms as nausea, vomiting,
abdominal pain, and bloating.24
Preliminary evidence suggests that patients with the irritable bowel
syndrome have increased serotonin levels in plasma and in the
rectosigmoid colon.25,26
Other neurotransmitters that may have an important role in functional
gastrointestinal disorders include calcitonin gene–related peptide,
acetylcholine, substance P, pituitary adenylate cyclase–activating polypeptide,
nitric oxide, and vasoactive intestinal peptide. These
neurotransmitters may provide links not only between bowel contractility
and visceral sensitivity, but also between the enteric and central
nervous systems.
Infection and Inflammation
There is compelling evidence that inflammation of the enteric
mucosa or neural plexuses initiates or contributes to symptoms associated
with irritable bowel syndrome.27,28,29
Mucosal inflammatory cytokines may activate peripheral sensitization
or hypermotility. Gwee et al. reported that in patients with
infectious enteritis, the presence of hypochondriasis and stressful
life events at the time of the acute infection predicted the
subsequent development of the irritable bowel syndrome.30
To date, no single conceptual model can explain all cases of the
syndrome.
Diagnosis
After a complete history has been obtained, all patients with
lower gastrointestinal tract symptoms should undergo a complete physical
examination and laboratory testing, including a complete blood
count, blood-chemistry tests, liver-function tests, and measurement
of thyrotropin. The diagnosis of the irritable bowel syndrome is
suggested when a patient's symptoms meet the Rome criteria. In the
majority of cases, there are no abnormalities on physical
examination or laboratory testing and there are no findings
suggestive of a structural disorder — so-called alarm symptoms.
Therefore, the irritable bowel syndrome can be reasonably diagnosed
on the basis of flexible sigmoidoscopy alone, in patients who are
less than 50 years old, or colonoscopy, in those who are 50 or
older; barium enema and flexible sigmoidoscopy are often substituted
for colonoscopy. In patients with diarrhea, a biopsy specimen should
be obtained from the mucosa of the descending colon to rule out microscopic
colitis.
If there are abnormalities on physical examination or laboratory
testing or if an alarm symptom is present, the irritable bowel syndrome
is a diagnosis of exclusion after reasonable diagnostic testing has
been performed, such as colonoscopy, computed tomographic scanning
of the abdomen and pelvis, and radiographic evaluation of the small
intestine. Alarm symptoms include evidence of gastrointestinal bleeding
such as occult blood in the stool, rectal bleeding, or anemia;
anorexia or weight loss; fever; persistent diarrhea causing
dehydration; severe constipation or fecal impaction; a family
history of gastrointestinal cancer, inflammatory bowel disease, or
celiac sprue; and the onset of symptoms at the age of at least 50
years. In 1985, the American College of Physicians recommended the
use of alarm symptoms to guide treatment31;
however, the validity of this approach has never been established in
rigorous, randomized, prospective clinical studies.
A number of structural or metabolic disorders that are responsive
to specific treatment cause symptoms similar to those of the irritable
bowel syndrome. Lactase deficiency is a common culprit. Other such
disorders include cancer of the colon, diverticulitis, mechanical
obstruction of the colon or small intestine, inflammatory bowel
disease, enteric infection, ischemia, maldigestion or malabsorption,
and endometriosis (suggested by the presence of pelvic pain at the
time of the menstrual period). In the absence of alarm symptoms, the
presence of one of these structural or metabolic disorders is very
unlikely.
Treatment
Whether the irritable bowel syndrome is diagnosed on the basis
of the history, physical examination, and laboratory tests or after
extensive testing (because of the presence of an alarm symptom), the
establishment of trust in the physician–patient relationship should
be given a high priority in order to maximize the efficacy of treatment
and minimize "doctor shopping." A diary of food intake and
symptoms can be useful in identifying foods that may be associated
with symptoms of the irritable bowel syndrome. Patients often report
an exacerbation of symptoms after the consumption of certain foods.
Some patients benefit from avoiding or limiting their intake of
caffeine, alcohol, fatty foods, gas-producing vegetables, or
products containing sorbitol, such as sugarless gum and dietetic
candy. The avoidance of constipating foods and the addition of 20 to
30 g of fiber per day either in the diet or in the form of
supplements such as bran, polycarbophil, or a psyllium derivative
may help relieve constipation32,33,34,35
and may occasionally improve diarrhea.
A rational approach to treating the irritable bowel syndrome uses
the patient's symptoms as a guide (Table 1). For
patients in whom the disorder is manifested predominantly as
abdominal pain, a variety of medications have been used, and several
new agents are under development. Antispasmodic agents may reduce
abdominal pain or bloating through anticholinergic pathways; in
refractory cases, nitrates are occasionally useful for direct relaxation
of smooth muscles. A recent meta-analysis suggested the
effectiveness of antispasmodic agents and tricyclic compounds in
treating selected patients with the irritable bowel syndrome.36
Unfortunately, many of these agents are not available in the United
States, and large-scale, stand-alone studies have not been
performed. Small studies have shown that tricyclic compounds in low
doses relieve unexplained abdominal pain.37
Side effects — sedation, dry mouth and eyes, and weight gain — limit
the use of primary tricyclic amines. Secondary tricyclic amines such
as nortriptyline and desipramine may be less likely to have side
effects.38
|
Despite speculation that 5-hydroxytryptamine (5-HT), or serotonin, receptors
may be involved in the pathogenesis of the irritable bowel syndrome,
the results of treatment with selective serotonin-reuptake inhibitors
have been disappointing. Nevertheless, serotonin has been implicated
in the modulation of visceral nociception, especially through the
5-HT3 and 5-HT4 pathways. Two new agents —
alosetron, a 5-HT3–receptor antagonist, and tegaserod, a
5-HT4 agonist — have been shown to diminish visceral sensitivity
to rectal distention in women who have diarrhea as the predominant symptom
of the irritable bowel syndrome and in those who have constipation
as the predominant symptom, respectively.39,40
Fedotozine, a kappa-opioid agonist,41
has shown promise as a visceral antinociceptive agent, and other
kappa-opioid agonists are being developed. Finally, in some patients
who have abdominal pain that is refractory to all these therapeutic
agents, treatment with classic analgesics such as nonsteroidal
antiinflammatory agents (perhaps with an initial trial of a
cyclooxygenase-2 inhibitor) or, in extreme cases, opioid analogues
may control the pain and improve the quality of life. The addictive
potential of opioid analogues makes them the last choice for
long-term therapy.
For patients in whom diarrhea is the predominant manifestation
of the irritable bowel syndrome, classic antidiarrheal agents such
as loperamide42
and diphenoxylate may help decrease the frequency of bowel movements
and improve the consistency of stool. In a study of women with this
form of the irritable bowel syndrome, alosetron prolonged colonic
transit, reduced the frequency of bowel movements and the urge to
defecate, improved the consistency of stool, and decreased abdominal
pain.43
However, this drug has been removed from the market because of side
effects such as severe constipation, ischemic colitis, and bowel
perforation. In cases of diarrhea that cannot be controlled,
cholestyramine has been used to bind bile acids that may be
responsible for increased secretion and decreased absorption of
water in the colon.44
In some refractory cases, a short course of antibiotics may reduce
the diarrhea, presumably by altering the intestinal flora.45
For patients in whom constipation is the predominant manifestation
of the irritable bowel syndrome, consumption of fiber may alleviate constipation
and related symptoms such as abdominal pain, tenesmus, and
dyschezia.32
Constipation can also be safely treated with osmotic laxatives such
as nonabsorbable carbohydrates (lactulose and sorbitol), milk of
magnesia or magnesium citrate, or a polyethylene glycol solution.
Two new classes of compounds, aminoguanidine indoles such as
tegaserod and benzofurans such as prucalopride, act specifically on
5-HT4 receptors. These agents shorten the transit time in
the colon and small intestine, increase the frequency of bowel
movements, and increase the softness of stools.46,47,48,49
Studies of prucalopride have been suspended because of concern about
tumorigenic effects in animals. Derivatives of anthraquinones (senna
and cascara), which act as strong laxatives, may be used as a last
resort, but their use is limited by the frequent development of
tachyphylaxis. The question of whether anthraquinones and their
derivatives damage the enteric nervous system has not been resolved.
Although many pharmacologic agents have been used to treat the
irritable bowel syndrome, few have been tested in controlled, double-blind
studies with adequate statistical power. The doses of some commonly
used agents are shown in Table 2.
|
The interaction between psychosocial factors and the genesis of all
forms of the irritable bowel syndrome is poorly understood. The most
provocative observations in this respect are that persons with the syndrome
who seek care are those in whom the syndrome is accompanied by a
psychiatric disorder and that the syndrome may develop in patients
who have both infectious gastroenteritis and a psychiatric disorder.
The potential benefits of supportive therapy, relaxation exercises,
hypnosis, cognitive behavioral therapy, and psychodynamic
interpersonal psychotherapy are well recognized.50
The irritable bowel syndrome is a common disorder that has a pronounced
effect on the quality of life and that accounts for a large
proportion of health care costs. Common pitfalls in diagnosing and
treating this disorder include unnecessary repetition of tests,
failure to establish trust in the physician–patient relationship,
and failure to provide the patient with realistic expectations
regarding the efficacy of medications. A concise diagnostic
evaluation and prompt institution of symptom-guided therapy can help
alleviate the pain and suffering experienced by patients with the
irritable bowel syndrome.
Source Information
From the Gastroenterology Section and the Functional
Gastrointestinal Diseases Center, Temple University School of Medicine,
Philadelphia.
Address reprint requests to Dr. Fisher at the Gastroenterology
Section, Temple University Hospital, 3401 N. Broad St., Philadelphia, PA 19140.
References
- Cumming
W. Electrogalvinism in a particular affliction of mucous membrane of the
bowels. London Med Gaz 1948;59:969-73.
- Thompson
WG, Longstreth G, Drossman DA. Functional bowel disorders and functional
abdominal pain. In: Drossman DA, Corazziari E, Talley N, Thompson WG,
Whitehead W, eds. The functional gastrointestinal disorders: diagnosis,
pathophysiology, and treatment. 2nd ed. McLean, Va.: Degnon, 2000:351-75.
- Camilleri
M, Choi M-G. Irritable bowel syndrome. Aliment Pharmacol Ther 1997;11:3-15.
- Everhart
JE, Renault PF. Irritable bowel syndrome in office-based practice in the
United States. Gastroenterology 1991;100:998-1005.
[Abstract]
- Drossman
DA, Whitehead WE, Camilleri M. Irritable bowel syndrome: a technical
review for practice guideline development. Gastroenterology
1997;112:2120-2137.
[Medline]
- Drossman
DA, Creed FH, Fava GA, et al. Psychosocial aspects of the functional
gastrointestinal disorders. Gastroenterol Int 1995;8:47-90.
- Azpiroz
F, Dapoigny M, Pace F, et al. Nongastrointestinal disorders in the
irritable bowel syndrome. Digestion 2000;62:66-72.
[Medline]
- Veale D,
Kavanagh G, Fielding JF, Fitzgerald O. Primary fibromyalgia and the
irritable bowel syndrome: different expressions of a common pathogenetic
process. Br J Rheumatol 1991;30:220-222.
[Medline]
- Talley
NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in
community subjects with irritable bowel syndrome. Gastroenterology
1995;109:1736-1741.
[Abstract]
- Almy TP.
Experimental studies on the irritable colon. Am J Med 1951;10:60-67.
- Snape WJ
Jr, Carlson GM, Matarazzo SA, Cohen S. Evidence that abnormal myoelectrical
activity produces colonic motor dysfunction in the irritable bowel
syndrome. Gastroenterology 1977;72:383-387.
[Abstract]
- Kumar D,
Wingate DL. The irritable bowel syndrome: a paroxysmal motor disorder.
Lancet 1985;2:973-977.
[Medline]
- Kellow
JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome
is correlated with symptoms. Gastroenterology 1987;92:1885-1893.
[Abstract]
- Whitehead
WE, Engel BT, Schuster MM. Irritable bowel syndrome: physiological and
psychological differences between diarrhea-predominant and
constipation-predominant patterns. Dig Dis Sci 1980;25:404-413.
[Medline]
- Kellow
JE, Phillips SF, Miller LJ, Zinsmeister AR. Dysmotility of the small
intestine in irritable bowel syndrome. Gut 1988;29:1236-1243.
[Abstract]
- Bonaz BL,
Papillon E, Baciu M, et al. Central processing of rectal pain in IBS
patients: an fMRI study. Gastroenterology 2000;118:Suppl:A615-A615.abstract
- Mertz H,
Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel
syndrome and control subjects with painful and nonpainful rectal
distension. Gastroenterology 2000;118:842-848.
[Medline]
- Ringel
Y, Drossman DA, Turkington TG, et al. Dysfunction of the motivational-affective
pain system in patients with IBS: pet brain imaging in response to rectal
balloon distension. Gastroenterology 2000;118:Suppl:A444-A444.abstract
- Silverman
DHS, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional
cerebral activity in normal and pathological perception of visceral pain.
Gastroenterology 1997;112:64-72.
[Abstract]
- Almy TP,
Kern F Jr, Tulin M. Alterations in colonic function in man under stress.
II. Experimental production of sigmoid spasm in healthy persons.
Gastroenterology 1949;12:425-436.
- Drossman
DA, McKee DC, Sandler RS, et al. Psychosocial factors in the irritable
bowel syndrome: a multivariate study of patients and nonpatients with
irritable bowel syndrome. Gastroenterology 1988;95:701-708.
[Abstract]
- Whitehead
WE, Bosmajian L, Zonderman AB, Costa PT Jr, Schuster MM. Symptoms of
psychologic distress associated with irritable bowel syndrome: comparison
of community and medical clinic samples. Gastroenterology 1988;95:709-714.
[Abstract]
- Drossman
DA. Sexual and physical abuse and gastrointestinal illness. Scand J
Gastroenterol Suppl 1995;208:90-96.
[Medline]
- Gershon
MD. Roles played by 5-hydroxytryptamine in the physiology of the bowel.
Aliment Pharmacol Ther 1999;13:Suppl 2:15-30.
- Bearcroft
CP, Perrett D, Farthing MJG. Postprandial plasma 5-hydroxytryptamine in
diarrhoea predominant irritable bowel syndrome: a pilot study. Gut
1998;42:42-46.
[Medline]
- Bose M,
Nickols C, Feakins R, Farthing MJ. 5-Hydroxytryptamine and
enterochromaffin cells in the irritable bowel syndrome. Gastroenterology
2000;118:Suppl:A563-A563.abstract
- Stewart
GT. Post-dysenteric colitis. BMJ 1950;1:405-409.
- Chaudhary
NA, Truelove SC. The irritable colon syndrome: a study of the clinical
features, predisposing causes, and prognosis in 130 cases. Q J Med
1962;31:307-322.
- McKendrick
MW, Read NW. Irritable bowel syndrome -- post salmonella infection. J
Infect 1994;29:1-3.
[Medline]
- Gwee KA,
Leong Y-L, Graham C, et al. The role of psychological and biological
factors in postinfective gut dysfunction. Gut 1999;44:400-406.
[Medline]
- Health
and Public Policy Committee, American College of Physicians. Endoscopy in
the evaluation of dyspepsia. Ann Intern Med 1985;102:266-269.
[Medline]
- Cann PA,
Read NW, Holdsworth CD. What is the benefit of coarse wheat bran in
patients with irritable bowel syndrome? Gut 1984;25:168-173.
[Abstract]
- Toskes
PP, Connery KL, Ritchey TW. Calcium polycarbophil compared with placebo in
irritable bowel syndrome. Aliment Pharmacol Ther 1993;7:87-92.
[Medline]
- Jalihal
A, Kurian G. Ispaghula therapy in irritable bowel syndrome: improvement in
overall well-being is related to reduction in bowel dissatisfaction. J
Gastroenterol Hepatol 1990;5:507-513.
[Medline]
- Prior A,
Whorwell PJ. Double blind study of ispaghula in irritable bowel syndrome.
Gut 1987;28:1510-1513.
[Abstract]
- Jailwala
J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel
syndrome: a systematic review of randomized, controlled trials. Ann Intern
Med 2000;133:136-147.
[Medline]
- Rajagopalan
M, Kurian G, Jacob J. Symptom relief with amitriptyline in the irritable
bowel syndrome. J Gastroenterol Hepatol 1998;13:738-741.
[Medline]
- Clouse
RE. Psychotropic medications for the treatment of functional
gastrointestinal disorders. Clin Perspect Gastroenterol 1999;2:348-56.
- Miura M,
Lawson DC, Clary EM, Mangel AW, Pappas TN. Central modulation of rectal
distension-induced blood pressure changes by alosetron, a 5-HT3 receptor
antagonist. Dig Dis Sci 1999;44:20-24.
[Medline]
- Schikowski
A, Mathis C, Thewissen M, Ross H-G, Pak MA, Enck P. Dose-dependent
modulation of rectal afferent sensitivity by a 5-HT4 receptor agonist.
Gastroenterology 1999;116:A643-A643.abstract
- Dapoigny
M, Abitbol JL, Fraitag B. Efficacy of peripheral kappa agonist fedotozine
versus placebo in treatment of irritable bowel syndrome: a multicenter
dose-response study. Dig Dis Sci 1995;40:2244-2249.
[Medline]
- Cann PA,
Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in
management of irritable bowel syndrome (IBS). Dig Dis Sci 1984;29:239-247.
[Medline]
- Camilleri
M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and
safety of alosetron in women with irritable bowel syndrome: a randomised,
placebo-controlled trial. Lancet 2000;355:1035-1040.
[Medline]
- Sciarretta
G, Fagioli G, Furno A, et al. 75Se HCAT test in the detection of bile acid
malabsorption in functional diarrhoea and its correlation with small bowel
transit. Gut 1987;28:970-975.
[Abstract]
- Pimentel
M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth
reduces symptoms of irritable bowel syndrome. Am J Gastroenterol
2000;95:3503-3506.
[Medline]
- Prather
CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod
accelerates orocecal transit in patients with constipation-predominant
irritable bowel syndrome. Gastroenterology 2000;118:463-468.
[Medline]
- Mueller-Lissner
S, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT4 receptor
partial agonist, relieves key symptoms of irritable bowel syndrome (IBS).
Gastroenterology 2000;118:Suppl:A175-A175.abstract
- Johanson
JF, Miner PB, Parkman HP, et al. Prucalopride (PRU) improves bowel
movement (BM) frequency and symptoms (SX) in patients (PTS) with chronic
constipation (CC): results of two double-blind, placebo-controlled trials.
Gastroenterology 2000;118:Suppl:A175-A175.abstract
- Sloots
CEJ, Poen AC, Felt-Bersma RJF, et al. Effects of prucalopride (PRU) on
colonic transit in patients (PTS) with chronic constipation (CC).
Gastroenterology 2000;118:Suppl:A847-A847.abstract
- Drossman
DA, Thompson WG. The irritable bowel syndrome: review and a graduated
multicomponent treatment approach. Ann Intern Med 1992;116:1009-1016.
[Medline]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.