|
|
Helicobacter pylori Infection and the Development of Gastric Cancer
Naomi Uemura, M.D., Shiro Okamoto, M.D., Soichiro Yamamoto,
M.D., Nobutoshi Matsumura, M.D., Shuji Yamaguchi, M.D., Michio Yamakido, M.D.,
Kiyomi Taniyama, M.D., Naomi Sasaki, M.D., and Ronald J. Schlemper, M.D.
|
|
ABSTRACT
Background Although
many studies have found an association between Helicobacter pylori infection and the
development of gastric cancer, many aspects of this relation remain
uncertain.
Methods We
prospectively studied 1526 Japanese patients who had duodenal
ulcers, gastric ulcers, gastric hyperplasia, or nonulcer dyspepsia
at the time of enrollment; 1246 had H.
pylori infection and 280 did not. The mean follow-up was
7.8 years (range, 1.0 to 10.6). Patients underwent endoscopy with
biopsy at enrollment and then between one and three years after
enrollment. H. pylori
infection was assessed by histologic examination, serologic testing,
and rapid urease tests and was defined by a positive result on any
of these tests.
Results Gastric
cancers developed in 36 (2.9 percent) of the infected and none of
the uninfected patients. There were 23 intestinal-type and 13
diffuse-type cancers. Among the patients with H. pylori infection, those with severe
gastric atrophy, corpus-predominant gastritis, and intestinal
metaplasia were at significantly higher risk for gastric cancer. We
detected gastric cancers in 21 (4.7 percent) of the 445 patients
with nonulcer dyspepsia, 10 (3.4 percent) of the 297 with gastric
ulcers, 5 (2.2 percent) of the 229 with gastric hyperplastic polyps,
and none of the 275 with duodenal ulcers.
Conclusions Gastric
cancer develops in persons infected with H. pylori but not in uninfected persons. Those with
histologic findings of severe gastric atrophy, corpus-predominant
gastritis, or intestinal metaplasia are at increased risk. Persons
with H. pylori
infection and nonulcer dyspepsia, gastric ulcers, or gastric
hyperplastic polyps are also at risk, but those with duodenal ulcers
are not.
Since the discovery of Helicobacter pylori in 1983,1 the
diagnosis and treatment of upper gastrointestinal disease have
changed greatly. A higher risk of the development of gastric cancer
has been reported in subjects with positive serologic tests for
H. pylori.2,3,4 The
World Health Organization and International Agency for Research on
Cancer consensus group5
stated in 1994 that there was sufficient epidemiologic and
histologic6,7
evidence to classify H. pylori
as a definite carcinogen. Most but not all recent studies8,9
have found H. pylori to be
associated with gastric cancer. The rates of infection in patients
with gastric cancer vary greatly among studies. These variations
may be attributable to differences in the methods of detecting H. pylori or in the patient groups. Most
prospective studies8,9
have used control groups "nested" within cohorts of study
patients from whom blood samples were taken years before the onset
of clinical gastric cancer. Various diagnostic tests for H. pylori10,11
may have false negative results, and the use of multiple tests may
help to provide a more accurate diagnosis of H.
pylori infection.12
In Japan, where the incidence of gastric cancer is high, endoscopy is
performed frequently for the early detection of gastric cancer, even
as part of the examination of patients without symptoms of the
disease. As a result, early-stage cancers are often discovered by
endoscopy.
We conducted a prospective, long-term study of a large group of
patients who were assessed for H. pylori
infection by endoscopy and biopsy, followed by histologic
examination, a rapid urease test, and serologic testing, to
determine the relation between H.
pylori infection and the development of gastric cancer.
Methods
Patients
Between April 1990 and March 1993, we enrolled 1603 consecutive
patients with active duodenal ulcers, active gastric ulcers, gastric
hyperplastic polyps, or nonulcer dyspepsia. They were assessed for H. pylori infection and underwent endoscopic
follow-up for the early detection of gastric cancer. We had
previously excluded patients with severe underlying disease,
including gastric cancer and adenoma, those who had undergone
gastric resection, and those taking nonsteroidal antiinflammatory drugs.
We then excluded 77 patients who declined a second endoscopic examination.
The remaining 1526 patients (869 men and 657 women; mean age, 52
years; range, 20 to 76) were studied. Endoscopy with biopsy was
performed in all patients at enrollment and between one and three
years after enrollment. Follow-up data were censored because of
deaths from other causes and the use of antibiotic treatment for the
eradication of H. pylori. The
mean duration of follow-up was 7.8 years (range, 1.0 to 10.6). All
patients gave written informed consent. The study protocol was
approved by the ethics committees of Kure Kyosai Hospital and was
reviewed annually.
Endoscopy and Histologic Examination
All endoscopic examinations were performed with only local
anesthesia (lidocaine). An Olympus videoscope (model GIF-230,
Olympus, Tokyo, Japan) was used. Four biopsy specimens were taken,
two from the greater curvature of the antrum and two from the upper
body of the stomach (when lesions suspected to be cancerous were
noted, additional biopsies were performed). Of these four specimens,
two were fixed in formalin and assessed for H.
pylori (by Giemsa staining) and the degree of neutrophil
infiltration and intestinal metaplasia (by staining with hematoxylin
and eosin). The remaining two were used for a rapid urease test
(CLO, Delta West, Bentley, Australia). The degree of neutrophil infiltration
was classified according to four grades (0 denoting no infiltration,
1 mild, 2 moderate, and 3 marked) and expressed as a score by two
pathologists according to the updated Sydney system.13
Consensus was reached through joint review of all the slides. Active
gastritis was classified into four categories (no gastritis,
antrum-predominant gastritis, pangastritis, and corpus-predominant
gastritis). Intestinal metaplasia was classified in two grades
(absent or present), because the multifocal distribution of
metaplasia may lead to misclassification when only two biopsy specimens
are obtained. Gastric mucosal atrophy was evaluated according to the
endoscopic-atrophic-border scale described by Kimura and Takemoto,14
which correlates with the results of histologic evaluation.15,16
There were three classifications (1 denoting mild atrophy or none, 2
moderate, and 3 severe). The pathologists were not aware of the
clinical or endoscopic data. The results were scored blindly with
the use of patient codes.
The rapid urease test was monitored for up to 24 hours. Gastric
cancer was defined as evident invasion of neoplastic epithelium into
the lamina propria of the mucosa or beyond (i.e., category 5.1 or
5.2 according to the Vienna classification17)
and was classified according to Laurén18
as intestinal or diffuse type.
Serologic Evaluation
Blood was sampled immediately before endoscopy; serum was
immediately separated and cryopreserved at –20°C until it was assayed
for antibodies against H. pylori
(HM-CAP, Enteric Products, Westbury, N.Y.). A positive serologic
test for H. pylori was defined
as one with a titer of 1.8 or more.
Detection of H. pylori Infection
H. pylori infection was identified by histologic
examination, the rapid urease test, and serologic evaluation.
Patients in whom any of these assays were positive were classified
as H. pylori–positive.
Those in whom all three were negative were considered H. pylori–negative.
Statistical Analysis
All statistical analyses were performed with SAS software (SAS
Institute, Cary, N.C.).19
The demographic and clinical characteristics of the patients were
compared by Student's t-test (for age, duration of follow-up, and
number of endoscopic procedures) or the chi-square test (for sex,
diagnosis, grade of gastric mucosal atrophy, distribution of
gastritis, and presence or absence of intestinal metaplasia). We
calculated relative risks for gastric findings — such as the degree
of atrophy, the pattern of distribution of gastritis, and the
presence of intestinal metaplasia — using Cox proportional-hazards
models. Since gastric cancer has not been demonstrated to develop in
patients with duodenal ulcers or in those who are H. pylori–negative (with or without eradication
therapy), we could not calculate the difference in the incidence of
gastric cancer using the Cox proportional-hazards model. For this
reason, Kaplan–Meier analysis and the chi-square test or Fisher's
exact test were used to assess the difference in proportions. All P
values are two-sided; significance was indicated by a P value of
less than 0.05.
Results
Of the 1526 patients, 1246 were H.
pylori–positive and 280 H.
pylori–negative. The base-line characteristics of both
groups are shown in Table 1. There
were no significant differences between the two groups in age, sex,
or the mean number of endoscopic procedures. The H. pylori–positive patients
included 445 with nonulcer dyspepsia (206 men and 239 women; mean
age, 54 years; range, 22 to 76), 275 with duodenal ulcers (198 men
and 77 women; mean age, 48 years; range, 20 to 76), 297 with gastric
ulcers (226 men and 71 women; mean age, 52 years; range, 22 to 75),
and 229 with gastric polyps (84 men and 145 women; mean age, 56
years; range, 26 to 76). The H.
pylori–negative patients all had nonulcer dyspepsia. Atrophy
and intestinal metaplasia of any grade were found in 4 percent and 2
percent of H. pylori–negative
patients, respectively. Of the H.
pylori–negative group, only 2 percent had gastritis, all
antrum predominant. In the H. pylori–positive
group, 53 percent had moderate atrophy and 17 percent had severe atrophy.
Antrum-predominant gastritis was found in 56 percent, pangastritis
in 27 percent, and corpus-predominant gastritis in 17 percent of H. pylori–positive patients. Thirty-seven
percent had intestinal metaplasia. There were significant differences
in these variables between the groups (P<0.001 by the chi-square test).
The duration of follow-up in the H. pylori–positive
group was significantly shorter than in the uninfected group (P<0.001),
because 253 of 1246 infected patients received eradication therapy
at an early stage of follow-up.
|
Development of Gastric Cancer
During follow-up, gastric cancer developed in 36 of 1246 H. pylori–infected patients
(2.9 percent) but in none of the 280 uninfected patients (P<0.001).
All cancers were visible on endoscopy and were identified
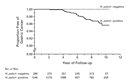
histologically on biopsy. In Figure 1, the
risk of gastric cancer is shown to be 5 percent at 10 years by
Kaplan–Meier analysis. There were 23 men and 13 women with gastric
cancer (at base line: mean age, 60 years; range, 41 to 76; at the
time of detection of gastric cancer: mean age, 65; range, 47 to 83).
Sixteen men and seven women had intestinal-type cancers (at base line:
mean age, 64 years; range, 44 to 76; at the time of detection of
gastric cancer: mean age, 70; range, 53 to 83), and six men and
seven women had diffuse-type cancers (at base line: mean age, 52
years; range, 41 to 68; at the time of detection of gastric cancer:
mean age, 58; range, 47 to 75). The mean age at enrollment and at
the time of detection of gastric cancer was significantly lower in
the patients with diffuse-type cancer than in those with
intestinal-type cancer (P<0.001 for both comparisons).
|
Table 2 shows
the abnormalities of the gastric mucosa at base line in all the H. pylori–infected patients and in the
36 patients with gastric cancer, as well as the relative risks of
cancer according to the base-line abnormalities. The frequency of
severe atrophy, corpus-predominant gastritis, and intestinal metaplasia
was significantly higher in patients with intestinal-type gastric
cancer than in those with diffuse-type cancer (P=0.002, P<0.001,
and P=0.008, respectively). Nine of the patients with diffuse-type
gastric cancer had moderate atrophy, and 10 had pangastritis.
|
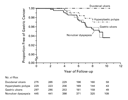
During follow-up, gastric cancer was detected in 21 of the 445 patients
with nonulcer dyspepsia (4.7 percent), in 10 of the 297 with gastric
ulcers (3.4 percent), and in 5 of the 229 with gastric polyps (2.2
percent) at base line (Figure 2). No
gastric cancer was detected in patients with duodenal ulcers. The
frequency of gastric cancer in patients with nonulcer dyspepsia,
gastric ulcers, and gastric polyps was significantly higher than in
those with duodenal ulcers (Table 3). The
frequency of diffuse-type cancer in patients with gastric ulcers was
significantly higher than in patients with nonulcer dyspepsia and
gastric polyps (P=0.03 by the chi-square test). The mean age at the
time of diagnosis of gastric cancer in patients with gastric ulcers
(53 years) was significantly lower than in those with nonulcer dyspepsia
(63 years) (P=0.009 by Student's t-test). Gastric cancer did not
develop in any of the 253 patients with H.
pylori infection who received eradication therapy. The
mean (±SD) duration of follow-up after eradication (4.8±1.2 years)
was shorter than the mean duration for patients who were not treated
(8.5±1.7 years; P<0.001 by Student's t-test).
|
|
Discussion
We found that gastric cancer developed in patients with H. pylori infection but not in
uninfected patients. Our findings are consistent with those of a
recent meta-analysis.8 In
Japan, it has been reported that each year, gastric cancer develops
in 300,000 (0.5 percent) of the 60 million people who are H. pylori–positive,20
which means that gastric cancer develops in 5 percent of H. pylori–positive persons over
10 years. Our results support this estimate.
In previous epidemiologic studies showing a close relation between
H. pylori infection and
gastric cancer, a large number of patients with negative serologic
results were found to have cancer.8,9
Recent studies10,11,12
have shown that false negative results occur with the serum antibody
assay, so it is possible that the rate of H. pylori infection has been underestimated in patients
with gastric cancer. Tabata et al.12
concluded from their study of this issue that a biopsy specimen
should be taken from the greater curvature of the upper gastric body
because this procedure results in fewer false negatives. Enomoto et
al.21
performed an immunohistologic study of biopsy specimens from the
greater curvature of the upper gastric body and antibodies against H. pylori; they found that 98
percent of patients with gastric cancer were H. pylori–positive. Their results and our
findings suggest that there are very few patients with gastric cancer
who are not infected with H. pylori.
It has previously been shown that in H. pylori–negative patients, histologic evidence
of gastritis, especially neutrophil infiltration, is rare, and
little gastric mucosal atrophy occurs.22,23
This is what we found as well. Thus, the onset of gastric cancer may
be related to histologic evidence of gastritis or atrophic gastritis
associated with H. pylori
infection.
Our findings suggest that patients with H. pylori infection and severe atrophic
gastritis, corpus-predominant gastritis, or both, along with
intestinal metaplasia are at high risk for intestinal-type gastric
cancer. It has been shown that intestinal-type gastric cancer
develops in patients who have severe atrophic gastritis in
association with intestinal metaplasia.24
Progression of atrophic gastritis can be caused by H. pylori infection.25
Our results confirm the hypothesis of Correa24
that severe atrophic gastritis accompanying intestinal metaplasia
caused by persistent H. pylori
infection is closely related to the development of intestinal-type
gastric cancer.
Since atrophic changes are not severe in diffuse-type gastric
cancer,25,26 it
was previously considered to have little relation to H. pylori infection. However,
epidemiologic and histopathological studies27,28
have shown that the development of diffuse-type cancer is also
closely related to H. pylori
infection. In our study, many of the patients with diffuse-type
gastric cancer had moderate atrophic changes and pangastritis. Our
results support the hypothesis of Sipponen et al.25
and Solcia et al.26
that diffuse-type gastric cancer develops during the progression of
atrophic gastritis in patients with H.
pylori infection and is associated particularly with
active gastritis.
In our study, gastric cancer developed in patients with nonulcer
dyspepsia, active gastric ulcers, and hyperplastic gastric polyps, but
no gastric cancers developed during follow-up in patients with
active duodenal ulcers. Hansson et al.29
have shown that gastric ulcer is associated with a high risk of
gastric cancer, whereas duodenal ulcer is associated with a low
risk. Patients with gastric ulcers typically have atrophic gastritis
and corpus-predominant gastritis. Patients with duodenal ulcers have
few atrophic changes and have antrum-predominant gastritis.30,31,32
Thus, there should be a higher rate of gastric cancer in patients
with gastric ulcers than in those with duodenal ulcers. Diffuse-type
gastric cancer is predominant in patients with gastric ulcers, many
of whom are relatively young. In young patients with gastric ulcers,
it is therefore necessary to perform careful follow-up to detect
diffuse-type gastric cancer even after ulcers have healed. No
gastric cancer developed after eradication of H.
pylori in 253 infected patients in our study, although the
duration of follow-up was relatively short. We have previously shown
that in patients with early gastric cancer that is treated by endoscopic
mucosal resection, eradication of H. pylori
prevents the development of new cancer or the continued growth of
occult cancer (i.e., cancer undetectable by endoscopy at the time of
initial treatment).33
In conclusion, we found that H.
pylori infection is associated with the development of
both intestinal-type and diffuse-type gastric cancer. Among infected
patients, those with severe atrophy accompanying intestinal
metaplasia, corpus-predominant gastritis, or both are at
particularly high risk.
Supported in part by a grant-in-aid for cancer research (8-14)
from the Ministry of Health and Welfare of Japan.
Presented in part at the annual meeting of the American
Gastroenterology Association, San Diego, Calif., May 20, 2000.
We are indebted to Professor Anthony Axon (of the Center for Digestive
Disease at the General Infirmary at Leeds, United Kingdom) and to
Professor Manfred Stolte (of the Institute of Pathology, Klinikum
Bayreuth, Germany) for their helpful suggestions and to Ms. Masako
Hiramatsu and Ms. Chiyo Maruyama for their excellent technical
assistance in the endoscopy unit.
Source Information
From the Departments of Gastroenterology (N.U., S.O., S.Y., N.M.,
S.Y., M.Y.) and Clinical Pathology (K.T., N.S.), Kure Kyosai Hospital, Kure
City; and the Department of Internal Medicine, Fukuoka University School of
Medicine, Fukuoka (R.J.S.) — both in Japan.
Address reprint requests to Dr. Uemura at the Department of
Gastroenterology, Kure Kyosai Hospital, 2-3-28 Nishi-chuo, Kure City, Japan, or
at [log in to unmask].
References
- Unidentified
curved bacilli on gastric epithelium in active chronic gastritis. Lancet
1983;1:1273-1275.
[Medline]
- Parsonnet
J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and
the risk of gastric carcinoma. N Engl J Med 1991;325:1127-1131.
[Abstract]
- Nomura
A, Stemmermann GN, Chyou P-H, Kato I, Perez-Perez GI, Blaser MJ.
Helicobacter pylori infection and gastric carcinoma among Japanese
Americans in Hawaii. N Engl J Med 1991;325:1132-1136.
[Abstract]
- Forman
D, Newell DG, Fullerton F, et al. Association between infection with
Helicobacter pylori and risk of gastric cancer: evidence from a
prospective investigation. BMJ 1991;302:1302-1305.
[Medline]
- Infection
with Helicobacter pylori. In: IARC monographs on the evaluation of the
carcinogenic risks to humans. Vol. 61. Schistosomes, liver flukes and
Helicobacter pylori. Lyon, France: International Agency for Research on
Cancer, 1994:177-241.
- Correa
P, Fox J, Fontham E, et al. Helicobacter pylori and gastric carcinoma: serum
antibody prevalence in populations with contrasting cancer risks. Cancer
1990;66:2569-2574.
[Medline]
- Sipponen
P, Hyvarinen H. Role of Helicobacter pylori in the pathogenesis of
gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl
1993;196:3-6.
[Medline]
- Huang
J-Q, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between
Helicobacter pylori seropositivity and gastric cancer. Gastroenterology
1999;114:1169-1179.
[Abstract/Full Text]
- Danesh J.
Helicobacter pylori infection and gastric cancer: systematic review of the
epidemiological studies. Aliment Pharmacol Ther 1999;13:851-856.
[Medline]
- Miwa H,
Kikuchi S, Ohtaka K, et al. Insufficient diagnostic accuracy of imported
serological kits for Helicobacter pylori infection in Japanese population.
Diagn Microbiol Infect Dis 2000;36:95-99.
[Medline]
- Ohara S,
Kato M, Asaka M, Toyota T. Studies of 13C-urea breath test for diagnosis
of Helicobacter pylori infection in Japan. J Gastroenterol 1998;33:6-13.
- Tabata
H, Fuchigami T, Kobayashi H, et al. Helicobacter pylori and mucosal
atrophy in patients with gastric cancer: a special study regarding the
methods for detecting Helicobacter pylori. Dig Dis Sci 1999;44:2027-2034.
[Medline]
- Dixon
MF, Genta RM, Yardley JH, Correa P. Classification and grading of
gastritis: the updated Sydney System. Am J Surg Pathol 1996;20:1161-1181.
[Medline]
- Kimura
K, Takemoto T. Endoscopic atrophy border. Endoscopy 1969;1:1-3.
- Satoh K,
Kimura K, Taniguchi Y, et al. Distribution of inflammation and atrophy in
the stomach of Helicobacter pylori-positive and -negative patients with
chronic gastritis. Am J Gastroenterol 1996;91:963-969.
[Medline]
- Ito S,
Azuma T, Murakita H, et al. Profile of Helicobacter pylori cytotoxin
derived from two areas of Japan with different prevalence of atrophic
gastritis. Gut 1996;39:800-806.
[Abstract]
- Schlemper
RJ, Riddell RH, Kato Y, et al. The Vienna classification of
gastrointestinal epithelial neoplasia. Gut 2000;47:251-255.
[Abstract/Full Text]
- Laurén
P. The two histological main types of gastric carcinoma: diffuse and
so-called intestinal-type carcinoma: an attempt at a histo-clinical
classification. Acta Pathol Microbiol Scand 1965;64:31-49.
- SAS/STAT
software: changes and enhancement, through release 6.11. Cary, N.C.: SAS
Institute, 1996.
- Asaka M,
ed. Gastric cancer: Helicobacter pylori and gastroduodenal diseases.
Tokyo, Japan: Sentan Igakusha, 1999:116-26. (In Japanese.)
- Enomoto
H, Watanabe H, Nishikura K, Umezawa H, Asakura H. Topographic distribution
of Helicobacter pylori in the resected stomach. Eur J Gastroenterol
Hepatol 1998;10:473-478.
[Medline]
- Blaser
MJ. Hypothesis on the pathogenesis and natural history of Helicobacter
pylori-induced inflammation. Gastroenterology 1992;102:720-727.
[Abstract]
- Kuipers
EJ, Uyterlinde AM, Pena AS, et al. Long-term sequelae of Helicobacter
pylori gastritis. Lancet 1995;345:1525-1528.
[Medline]
- Correa
P. Human gastric carcinogenesis: a multistep and multifactorial process --
First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention.
Cancer Res 1992;52:6735-6740.
[Abstract]
- Sipponen
M, Kosunen TU, Valle J, Riihela M, Seppala K. Helicobacter pylori
infection and chronic gastritis in gastric cancer. J Clin Pathol
1992;45:319-323.
[Abstract]
- Solcia
E, Fiocca R, Luinetti O, et al. Intestinal and diffuse gastric cancers
arise in a different background of Helicobacter pylori gastritis through
different gene involvement. Am J Surg Pathol 1996;20:Suppl 1:S8-S22.
[Medline]
- Kikuchi
S, Wada O, Nakajima T, et al. Serum anti-Helicobacter pylori antibody and
gastric carcinoma among young adults. Cancer 1995;75:2789-2793.
[Medline]
- Kokkola
A, Valle J, Haapiainen R, Sipponen P, Kivilaakso E, Puolakkainen P.
Helicobacter pylori infection in young patients with gastric carcinoma.
Scand J Gastroenterol 1996;31:643-647.
[Medline]
- Hansson
L-E, Nyrén O, Hsing AW, et al. The risk of stomach cancer in patients with
gastric or duodenal ulcer disease. N Engl J Med 1996;335:242-249.
[Abstract/Full Text]
- Stolte
M, Eidt S, Ohnsmann A. Differences in Helicobacter pylori associated
gastritis in the antrum and body of the stomach. Z Gastroenterol
1990;28:229-233.
[Medline]
- Meining
A, Stolte M, Hatz R, et al. Differing degree and distribution of gastritis
in Helicobacter pylori-associated diseases. Virchows Arch 1997;431:11-15.
[Medline]
- Graham
DY. Helicobacter pylori: its epidemiology and its role in duodenal ulcer
disease. J Gastroenterol Hepatol 1991;6:105-113.
[Medline]
- Uemura
N, Mukai T, Okamoto S, et al. Effect of Helicobacter pylori eradication on
subsequent development of cancer after endoscopic resection of early
gastric cancer. Cancer Epidemiol Biomarkers Prev 1997;6:639-642.
[Abstract]
This article has been cited by other
articles:
- Fox, J.
G., Wang, T. C. (2001). Helicobacter pylori -- Not a Good Bug after All. N
Engl J Med 345: 829-832
[Full Text]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.