|
|
Essential Tremor
Elan D. Louis, M.D.
|
|
This Journal feature
begins with a case vignette highlighting a common clinical problem.
Evidence supporting various strategies is then presented, followed
by a review of formal guidelines, when they exist. The article ends
with the author's clinical recommendations.
A 66-year-old woman presents with tremor
of the hands. She first noticed a mild tremor six years earlier, but
the tremor has been worsening for the past two years. It occurs when
she is using her hands, interfering with some activities of daily
living. For example, she can no longer eat soup without spilling,
put in her contact lenses, or apply lipstick. A writer, she is no
longer able to write in longhand or type her manuscripts and must
dictate them. The tremor causes a great deal of embarrassment in social
situations. How should this patient be evaluated and treated?
The Clinical Problem
The first step in evaluating any patient with tremor is to
characterize the tremor. All humans have physiologic tremor, or
rhythmic oscillatory movements, of their hands that is detectable
with the use of electrophysiological techniques such as quantitative
accelerometry. Stressful circumstances, such as those inducing fear,
anger, or fatigue, may cause transient enhanced physiologic tremor,
which may be visible to the naked eye.
In addition to these normal forms of tremor, there are several
pathologic tremors. They may take several forms: resting tremor, action
tremor (also referred to as akinetic or postural tremor), and
intention tremor. Resting tremor occurs while the limb is relaxed
and stationary — for example, in the hand while a person is standing
or walking. Action tremor occurs during sustained extension of the
arm or during voluntary motion such as writing or pouring. Intention
tremor is a coarse terminal tremor that occurs as the limb
approaches a target — for example, during the finger-to-nose
maneuver. It is often accompanied by ataxic gait and other signs of
cerebellar disease (e.g., nystagmus and slurred or scanning speech).
Action tremor is the most prevalent of these types of tremor and,
because it is present when the hands are in active use, is
disabling. The most common action tremor is essential tremor, a
tremor of the hands of 4 to 12 Hz. It may also affect the head,
voice, trunk, and legs. Estimates of the prevalence of essential
tremor in the general population range from 0.4 to 6 percent1
and are higher among persons over 65 years of age. Although
essential tremor is often referred to as benign, this description is
misleading. Between 15 and 25 percent of patients with essential
tremor who are seen in clinical practice retire prematurely, and 60
percent choose not to apply for a job or promotion because of the
uncontrollable shaking of their hands.2
Strategies and Evidence
Diagnosis
In addition to essential tremor, the differential diagnosis of
an action tremor includes enhanced physiologic tremor that is
sustained (as a result of either an identifiable cause, such as
medication or hyperthyroidism, or a cause that is not readily identifiable),
Parkinson's disease, adult-onset idiopathic dystonia, and Wilson's
disease (Table 1).
With the exception of essential tremor and enhanced physiologic
tremor, these other disorders are rare (prevalence, <1 percent).
The diagnostic approach includes obtaining a history, physical
examination, and laboratory tests.
|
During the history-taking, the age at onset, type, and rate of
progression of the tremor should be assessed, along with information
about family history (Table 1).
Caffeine, cigarettes, and medications such as lithium, prednisone,
levothyroxine, beta-adrenergic bronchodilators, valproate, and
selective serotonin-reuptake inhibitors commonly result in enhanced
physiologic tremor; a complete inventory of all current medications,
as well as caffeine intake and smoking habits, is important in order
to exclude these as the cause of tremor. Patients with tremor due to
other disorders usually have symptoms of these disorders at
presentation; for example, diarrhea or weight loss in patients with
hyperthyroidism, loss of normal facial expression or a change in
normal arm swing in patients with Parkinson's disease, the sensation
that muscles in the hand or neck are being pulled or twisted in
patients with dystonia, and reports of depression, cognitive
impairment, or other involuntary movements in patients with Wilson's
disease.
During the physical examination, the clinician should carefully
assess the characteristics of the tremor (Table 1).
Although many patients with Parkinson's disease have a mild postural
tremor,5
resting tremor is also present and affects approximately 85 percent
of patients in studies of autopsy-proved Parkinson's disease.6
Approximately 20 to 30 percent of patients with dystonia have a
postural tremor that superficially resembles essential tremor (Table 1). If
symptoms or signs of hyperthyroidism are present, then
thyroid-function tests should be performed.
In any patient with action tremor who is younger than 40 years
of age, the possibility of Wilson's disease should be explored with
a measurement of serum ceruloplasmin. The level is low (<20 mg
per deciliter) in 95 percent of patients with this disorder.7 If
other clinical features suggestive of Wilson's disease are present,
such as dysarthria, dystonia, and parkinsonism, then a careful
slit-lamp examination of the eye should be performed by an
experienced ophthalmologist. Kayser–Fleischer rings on Descemet's
membrane are detectable in 99.3 percent of patients with Wilson's
disease who have neurologic abnormalities.3
Quantitative computerized analysis of tremor is available at some
tertiary care centers and may guide the clinician in distinguishing essential
tremor from other types of tremor, but its diagnostic validity, like
that of positron-emission tomography, has not been established. At
present, the diagnosis of essential tremor is based on clinical findings;
there are no validated serologic, radiologic, or pathological
markers (videotaped examples of clinical findings in patients with
essential tremor are available as Supplementary
Appendix 1). There is no gold standard for diagnosis, but the
level of agreement is high between clinicians using the same
clinical criteria (Table 2).1,4
|
Treatment
Pharmacotherapy may be used to improve function or reduce the
embarrassment associated with essential tremor. Medications are
not indicated for mild cases that do not cause dysfunction or
embarrassment. Surgery is indicated for severe cases that are refractory
to medications. This discussion, with one exception,8
includes results only from double-blind, placebo-controlled trials,
but these have generally been small.
Two issues deserve special emphasis. First, although a reduction
in the amplitude of tremor, measured by clinical rating scales or
accelerometric methods, may be important, the ultimate evidence of
efficacy is a reduction in functional impairment. Second, although
in groups of patients these medications reduce the severity of
tremor, on an individual basis, a large proportion of patients (25
to 55 percent) have no response. A number of factors may be
responsible for this high rate of nonresponse. There is evidence
that essential tremor is not a homogeneous condition, and that
different clinical subtypes (e.g., young-onset essential tremor or
essential tremor with head tremor) may differ in pathogenesis and
response to treatment.9
The value of most current medications was discovered as a result of
serendipity rather than through an understanding of the mechanisms
of the disease, which remains limited.
First-Line
Therapies
Propranolol and primidone are the first-line agents in the
treatment of essential tremor (Table 3).
Peripheral ![]() -adrenergic receptors
probably mediate the effects of
-adrenergic receptors
probably mediate the effects of ![]() -adrenergic–blocking
agents,11
although central mechanisms may be involved as well. Several placebo-controlled
studies have demonstrated that propranolol (at a dose of at least
120 mg per day) results in a significant reduction in the severity
of tremor,12
and subjectively, 45 to 75 percent of patients report that
propranolol is more effective than placebo in reducing their tremor.
Although propranolol is well tolerated, relative contraindications
include asthma, congestive heart failure, diabetes mellitus, and
atrioventricular block. Propranolol, a nonselective antagonist, is
more effective than antagonists with relatively selective
-adrenergic–blocking
agents,11
although central mechanisms may be involved as well. Several placebo-controlled
studies have demonstrated that propranolol (at a dose of at least
120 mg per day) results in a significant reduction in the severity
of tremor,12
and subjectively, 45 to 75 percent of patients report that
propranolol is more effective than placebo in reducing their tremor.
Although propranolol is well tolerated, relative contraindications
include asthma, congestive heart failure, diabetes mellitus, and
atrioventricular block. Propranolol, a nonselective antagonist, is
more effective than antagonists with relatively selective ![]() 1 activity,11
and trials of
1 activity,11
and trials of ![]() 1-selective
agents (such as atenolol and metoprolol) have had mixed results.11,13
Long-acting propranolol is as effective as conventional propranolol,
and compliance with the long-acting formulation is significantly
better.14
1-selective
agents (such as atenolol and metoprolol) have had mixed results.11,13
Long-acting propranolol is as effective as conventional propranolol,
and compliance with the long-acting formulation is significantly
better.14
|
Primidone, an anticonvulsant medication, is metabolized to
phenylethylmalonamide and phenobarbitone. Although the barbiturate
metabolite may contribute to the therapeutic effect, the parent
compound is thought to mediate most of this effect.15,16,17
Primidone is superior to phenobarbital in reducing the severity of
tremor.18
Primidone in doses of up to 750 mg per day was significantly more
effective than placebo in reducing tremor,15,16,17
but tolerability is a common problem. Even at a low starting dose
(62.5 mg per day), an acute toxic reaction consisting of nausea, vomiting,
or ataxia has been reported in 23 percent15
to 73 percent16
of patients, requiring discontinuation of primidone in approximately
20 percent of patients in some studies.15
A 750-mg daily dose of primidone was compared with a 120-mg daily
dose of propranolol, and although each was significantly better than
placebo,17
neither was conclusively shown to be superior to the other in this
small study. The mean reduction in the amplitude of tremor was 76
percent while patients were taking primidone, as compared with a
reduction of 60 percent during treatment with propranolol, a
nonsignificant difference. More patients indicated a preference for
primidone than for propranolol.17
In another study, the amplitude of tremor was reduced by a mean of
60 percent in 9 patients who were taking primidone (250 mg per day),
as compared with a mean reduction of 35 percent in 22 patients who
were taking their maximally effective dose of propranolol (P value
not reported).19
Although initial tolerability is a problem with primidone, there
is some evidence that the long-term tolerability of primidone is
superior to that of propranolol. In a study of 25 patients with
essential tremor, acute adverse reactions occurred in 8 percent of
the propranolol group and 32 percent of the primidone group;
however, clinically significant long-term (one year) side effects
occurred in none of the patients in the primidone group, as compared
with 17 percent of those in the propranolol group.20
Second-Line
Therapies
Gabapentin, an anticonvulsant medication, is structurally similar
to the inhibitory neurotransmitter ![]() -aminobutyric
acid (GABA). There is some evidence that the GABA-ergic system is
disturbed in essential tremor.21
There have been three trials of gabapentin involving a total of 54
patients. In two of the three studies, gabapentin (1200 to 3600 mg
per day) reduced tremor significantly more than did placebo, and in
one of the two, its effect was similar to that of propranolol.22
Gabapentin is well tolerated.
-aminobutyric
acid (GABA). There is some evidence that the GABA-ergic system is
disturbed in essential tremor.21
There have been three trials of gabapentin involving a total of 54
patients. In two of the three studies, gabapentin (1200 to 3600 mg
per day) reduced tremor significantly more than did placebo, and in
one of the two, its effect was similar to that of propranolol.22
Gabapentin is well tolerated.
Benzodiazepines potentiate the effect of GABA by binding to the
GABAA receptor. Alprazolam is the only benzodiazepine shown to be
effective in essential tremor in controlled trials. In one
placebo-controlled trial, alprazolam (0.75 to 2.75 mg per day)
resulted in a significant reduction in tremor, and 75 percent of the
patients had at least some improvement; however, drowsiness or
sedation occurred in 50 percent of the patients.23
Clonazepam, another benzodiazepine, did not significantly reduce the
severity of tremor in a placebo-controlled trial.24
One concern with the use of benzodiazepines is the risk of
dependence.
Calcium-channel blockers have had variable rates of success. Trials
of flunarizine (10 mg per day), which is not available in the United
States, have reported mixed results. In one placebo-controlled trial,
flunarizine significantly reduced the severity of tremor, with 13 of
15 patients having an improvement. In a second trial, however, none
of the patients had a decrease in the severity of tremor.25
Differences in the severity of tremor and the use of concurrent
medications may have accounted for some of this variation. In one
placebo-controlled trial, nimodipine (30 mg per day) significantly
reduced the severity of tremor, with 8 of 15 patients having an
improvement.26
Some calcium-channel blockers, such as nifedipine,27
may worsen tremor.
Theophylline may enhance the sensitivity to GABA. Theophylline
(150 mg per day) was compared with propranolol (80 mg per day) and
placebo.10
Theophylline reduced tremor significantly more than did placebo, and
its effect was similar to that of propranolol. However, the
investigators acknowledged that although the trial was designed as a
double-blind study, the side effects experienced by the patients
meant that in practice the treatments could be distinguished.
Other
Therapies
Intramuscular injections of botulinum toxin A into the intrinsic
muscles of the dominant hand may reduce tremor by weakening the
muscles or by blocking gamma motor efferents and muscle spindle
afferents.28
Although there was a significant reduction in the amplitude of
tremor among patients receiving one to two such injections, no
significant improvement in function was observed.
Action tremor may be mediated by neuronal loops that pass from
the cerebellum to the cortex by way of the ventral intermediate nucleus
of the thalamus. Two surgical approaches to tremor reduction involve
continuous deep-brain stimulation through an electrode implanted in
the ventral intermediate nucleus of the thalamus and surgical
lesioning of this nucleus (thalamotomy) contralateral to the more
disabled arm. The two methods were compared in a prospective,
randomized, single-blind study.8
Both procedures were equally effective in reducing tremor. Thalamic
stimulation resulted in greater improvement in self-reported
measures of function and fewer adverse events, including cognitive
deterioration, dysarthria, and gait or balance disturbances.8
Thalamic stimulation is the surgery of choice, because there are
fewer adverse events and the clinician can adjust the stimulator
settings during follow-up care.
Areas of Uncertainty
The response to medications is variable, and factors that predict
a positive response have not been identified. It is often necessary to
try several agents sequentially. Some clinical trials have suggested
that the efficacy of medications is lower in patients with tremor of
the head than in those with tremor of the hands, although most have
not enrolled adequate numbers of patients with head tremor, so have
had limited power to detect changes in this subgroup.
Guidelines
There are no guidelines from the American Academy of Neurology
or the Movement Disorder Society on the diagnostic workup or treatment
of essential tremor.
Conclusions and Recommendations
Action tremor is commonly seen in clinical practice, and essential
tremor is the most frequent cause. There is no diagnostic laboratory
test for essential tremor; the diagnosis is based on the history and
physical examination. In persons younger than 40 years of age, the
possibility of Wilson's disease should be excluded. Treatment of
essential tremor should be reserved for patients who have functional
impairment or embarrassment, as is the case for the patient
described in the vignette. Both primidone and propranolol are
effective. There is some evidence that primidone may be more
effective and better tolerated than propranolol, although this needs
to be studied further. Therefore, I consider primidone to be a
reasonable first choice, and if it cannot be tolerated, then
propranolol is my second choice. Gabapentin and benzodiazepines are
alternatives, although the latter may result in sedation at the
doses required to treat tremor. There are too few data to support
the use of nimodipine and theophylline. If the patient has no
response to medications and is sufficiently disabled, then
unilateral implantation of a deep-brain thalamic stimulator
contralateral to the more disabled arm is another option in centers
where this is available.
Supported by a grant from the National Institutes of Health (R01
NS39422) and by a Paul Beeson Physician Faculty Scholars in Aging
Research Award.
Source Information
From the Gertrude H. Sergievsky Center and the Department of
Neurology, Columbia University, New York.
Address reprint requests to Dr. Louis at Unit 198, Neurological
Institute, 710 W. 168th St., New York, NY 10032, or at [log in to unmask].
References
- Louis
ED, Ford B, Frucht S, Barnes LF, X-Tang M, Ottman R. Risk of tremor and
impairment from tremor in relatives of patients with essential tremor: a
community-based family study. Ann Neurol 2001;49:761-769.
[Medline]
- Bain PG,
Findley LJ, Thompson PD, et al. A study of hereditary essential tremor.
Brain 1994;117:805-824.
[Abstract]
- Walshe
JM, Yealland M. Wilson's disease: the problem of delayed diagnosis. J
Neurol Neurosurg Psychiatry 1992;55:692-696.
[Abstract]
- Louis
ED, Wendt KJ, Pullman SL, Ford B. Is essential tremor symmetric?
Observational data from a community-based study of essential tremor. Arch
Neurol 1998;55:1553-1559.
[Medline]
- Koller
WC, Vetere-Overfield B, Barter R. Tremors in early Parkinson's disease.
Clin Neuropharmacol 1989;12:293-297.
[Medline]
- Louis
ED, Klatka LA, Liu Y, Fahn S. Comparison of extrapyramidal features in 31
pathologically confirmed cases of diffuse Lewy body disease and 34
pathologically confirmed cases of Parkinson's disease. Neurology
1997;48:376-380.
[Abstract]
- Scheinberg
IH, Sternlieb I. Wilson's disease. Philadelphia: W.B. Saunders, 1984.
- Schuurman
PR, Bosch DA, Bossuyt PMM, et al. A comparison of continuous thalamic
stimulation and thalamotomy for suppression of severe tremor. N Engl J Med
2000;342:461-468.
[Abstract/Full Text]
- Louis
ED, Ford B, Barnes LF. Clinical subtypes of essential tremor. Arch Neurol
2000;57:1194-1198.
[Medline]
- Mally J,
Stone TW. Efficacy of an adenosine antagonist, theophylline, in essential
tremor: comparison with placebo and propranolol. J Neurol Sci
1995;132:129-132.
[Medline]
- Jefferson
D, Jenner P, Marsden CD.
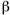 -Adrenoreceptor
antagonists in essential tremor. J Neurol Neurosurg Psychiatry
1979;42:904-909.
-Adrenoreceptor
antagonists in essential tremor. J Neurol Neurosurg Psychiatry
1979;42:904-909.[Abstract]
- Tolosa
ES, Loewenson RB. Essential tremor: treatment with propranolol. Neurology
1975;25:1041-1044.
[Abstract]
- Dietrichson
P, Espen E. Effects of timolol and atenolol on benign essential tremor:
placebo-controlled studies based on quantitative tremor recording. J
Neurol Neurosurg Psychiatry 1981;44:677-683.
[Abstract]
- Cleeves
L, Findley LJ. Propranolol and propranolol-LA in essential tremor: a
double blind comparative study. J Neurol Neurosurg Psychiatry
1988;51:379-384.
[Abstract]
- Findley
LJ, Cleeves L, Calzetti S. Primidone in essential tremor of the hands and
head: a double blind controlled clinical study. J Neurol Neurosurg
Psychiatry 1985;48:911-915.
[Abstract]
- Sasso E,
Perucca E, Fava R, Calzetti S. Primidone in the long-term treatment of
essential tremor: a prospective study with computerized quantitative
analysis. Clin Neuropharmacol 1990;13:67-76.
[Medline]
- Gorman
WP, Cooper R, Pocock P, Campbell MJ. A comparison of primidone,
propranolol, and placebo in essential tremor, using quantitative analysis.
J Neurol Neurosurg Psychiatry 1986;49:64-68.
[Abstract]
- Sasso E,
Perucca E, Calzetti S. Double-blind comparison of primidone and
phenobarbital in essential tremor. Neurology 1988;38:808-810.
[Abstract]
- Koller
WC, Royse VL. Efficacy of primidone in essential tremor. Neurology
1986;36:121-124.
[Abstract]
- Koller
WC, Vetere-Overfield B. Acute and chronic effects of propranolol and
primidone in essential tremor. Neurology 1989;39:1587-1588.
[Abstract]
- Louis
ED. A new twist for stopping the shakes? Revisiting GABAergic therapy for
essential tremor. Arch Neurol 1999;56:807-808.
[Medline]
- Ondo W,
Hunter C, Vuong KD, Schwartz K, Jankovic J. Gabapentin for essential
tremor: a multiple-dose, double-blind, placebo-controlled trial. Mov
Disord 2000;15:678-682.
[Medline]
- Huber
SJ, Paulson GW. Efficacy of alprazolam for essential tremor. Neurology
1988;38:241-243.
[Abstract]
- Thompson
C, Lang A, Parkes JD, Marsden CD. A double-blind trial of clonazepam in
benign essential tremor. Clin Neuropharmacol 1984;7:83-88.
[Medline]
- Curran
T, Lang AE. Flunarizine in essential tremor. Clin Neuropharmacol
1993;16:460-463.
[Medline]
- Biary N,
Bahou Y, Sofi MA, Thomas W, al Deeb SM. The effect of nimodipine on
essential tremor. Neurology 1995;45:1523-1525.
[Abstract]
- Topaktas
S, Onur R, Dalkara T. Calcium channel blockers and essential tremor. Eur
Neurol 1987;27:114-119.
- Jankovic
J, Schwartz K, Clemence W, Aswad A, Mordaunt J. A randomized,
double-blind, placebo-controlled study to evaluate botulinum toxin type A
in essential hand tremor. Mov Disord 1996;11:250-256.
[Medline]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.
