|
|
The Prevention of Pneumococcal Disease in
Children
G. Scott Giebink, M.D.
|
|
Otitis media in children leads to more than 20 million visits
to physicians annually in the United States.1,2,3 By
three years of age, 80 percent of all children in the United States
have had at least one episode of otitis media, and 50 percent have
had at least three episodes.2
Recurrent acute otitis media has its onset almost exclusively before
a child's second birthday.2
Streptococcus pneumoniae
(pneumococcus) is the most common cause of acute otitis media,
accounting for approximately 50 percent of all cases.4,5,6
In addition to being a cause of otitis media, S. pneumoniae remains a major
cause of childhood illness and death. At least 1 million children
die of pneumococcal infections (pneumonia, meningitis, and bacteremia)
each year, mostly in developing countries.7 A
meta-analysis of the outcomes of bacterial meningitis in developed
countries revealed that pneumococcal disease was associated with
higher rates of death (15 percent) and neurologic sequelae (12 to 28
percent) than either Haemophilus influenzae
or Neisseria meningitidis
infection.8
Studies in Finland9
and France10
suggest that 13 to 38 percent of community-acquired pneumonia in
children is caused by pneumococcus, a finding consistent with the
results of a controlled efficacy trial of pneumococcal conjugate
vaccine.11
Hence, pneumococcal pneumonia also represents a major disease burden
in children.
In February 2000, the Food and Drug Administration (FDA) licensed
a 7-valent pneumococcal conjugate vaccine (pneumococcal–CRM197, Wyeth
Lederle Vaccines, Pearl River, N.Y.), which is recommended for
routine use in infants. This vaccine, together with the identification
of risk factors for otitis media and pneumococcal colonization of
the nasopharynx, provides new opportunities for preventing and
managing pneumococcal disease. Given the increasing prevalence of
pneumococci with resistance to multiple antimicrobial drugs, these
new approaches should find immediate use.
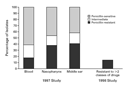
Pneumococcal Antibiotic Resistance
The overuse of antibiotics has contributed to the rapidly
increasing prevalence of drug-resistant S. pneumoniae, making it more complicated to
treat pneumococcal disease in young children successfully (Figure 1).
Antibiotics are prescribed empirically to virtually all children in
the United States who have acute otitis media. Yet 70 percent of
severe cases and 90 percent of mild cases of acute otitis media
resolve spontaneously with placebo treatment.14
Moreover, 10 to 30 percent of samples of middle-ear fluid from patients
with acute otitis media do not contain viable bacteria. Unfortunately,
it is difficult and time-consuming to sample middle-ear fluid by
tympanocentesis for culture, a practice that would eliminate
empiricism from the selection and use of antibiotics.
|
Among invasive isolates of pneumococcus collected from patients of
all ages in sentinel hospitals throughout the United States during
1991 and 1992, 6.6 percent were resistant to penicillin and 5.9
percent had multidrug resistance.15
By 1993 to 1994, 18.4 percent of the isolates collected from
children younger than six years old nationwide were resistant to
penicillin, and 13.1 percent were resistant to multiple antibiotics.16
In a 1997 study of 290 isolates collected from the middle ear of
children two years old or younger with acute otitis media throughout
the United States, the prevalence of strains that were highly resistant
to penicillin (49.7 percent) was higher than that of strains with an
intermediate level of resistance to penicillin (19.5 percent).12
The emergence of cross-resistance among antibiotics extends to
trimethoprim–sulfamethoxazole, macrolides, cephalosporins, and
clindamycin, and the prevalence of multidrug resistance continues to
increase.13
Risk Factors for Otitis Media and Nasopharyngeal
Carriage
Risk factors have been identified that predispose children to
otitis media and drug-resistant S.
pneumoniae disease. Male sex, attendance at a child-care
center outside the home, the absence of breast-feeding, passive
exposure to tobacco smoke, and having a sibling with a history of
recurrent otitis media all correlate with an increased risk of
otitis media in young children.2,17
As with many childhood infectious diseases, attendance at a
child-care center outside the home greatly increases the risk of
otitis media, any pneumococcal infection, and infection with
drug-resistant S. pneumoniae.18,19
Previous antibiotic treatment and an age of less than two years are
additional risk factors for otitis media caused by drug-resistant S. pneumoniae.20,21
The nasopharyngeal carriage of pneumococcus is highly prevalent
among young children and predisposes the carrier, his or her siblings,
and others in close contact with the carrier to pneumococcal infection.
The rates of nasopharyngeal carriage are 44 percent among all children
six years old or younger,22
60 to 80 percent among children attending child-care centers outside
the home,23,24,25
and more than 70 percent among children younger than three years of
age who have acute otitis media.26
Drug-resistant strains of S.
pneumoniae are highly prevalent among children colonized with
pneumococcus. In several studies, drug-resistant S. pneumoniae accounted for 37 to 53 percent of
these pneumococci, and the frequency of resistant strains was
highest in children younger than two years of age.22,23,24
Nasopharyngeal carriage of pneumococci is significantly associated
with the development of acute otitis media, and children colonized
with resistant strains are more likely than others to have
unresolved acute otitis media.22
Treatment of Acute Otitis Media
The widespread prescription of broad-spectrum antibiotics for
acute otitis media regardless of the pathogens involved contributes substantially
to the current trend toward increasing resistance to antimicrobial
drugs, as does the frequent use of antibiotics for nonbacterial pharyngitis
and bronchitis. Clinicians should educate parents regarding the
appropriate use of antibiotics and strategies to reduce the
likelihood of acute otitis media, including breast-feeding for at
least three months and eliminating children's exposure to tobacco
smoke. The judicious use of antibiotics requires an approach of
watchful waiting in cases in which the child is asymptomatic or has
red tympanic membrane but no middle-ear effusion.27
The use of tympanocentesis for the culture of middle-ear fluid in
patients with acute otitis media that is unresponsive to antibiotic
treatment would go far toward the more accurate determination of the
appropriate choice of antibiotic therapy.28
The antimicrobial drugs selected for children in whom first-line
treatment of acute otitis media with amoxicillin has failed must
meet two criteria — effectiveness against beta-lactamase–producing H. influenzae and Moraxella catarrhalis and effectiveness
against drug-resistant S.
pneumoniae.5
The latter requirement is the more restrictive, since recent data on
the eradication of drug-resistant S.
pneumoniae from the middle ear are lacking for most antibiotics.
High-dose amoxicillin (80 to 90 mg per kilogram of body weight per
day) plus clavulanate, cefuroxime axetil, and intramuscular ceftriaxone
meet these requirements in most cases, and clindamycin is often
effective when drug-resistant S. pneumoniae
has been proved by tympanocentesis to be the infecting organism.5
Vaccination to Prevent Pneumococcal Acute Otitis
Media
Clinicians have fewer treatment options today for pneumococcal
acute otitis media, given the increasing resistance to antibiotics, the
widespread nasopharyngeal carriage among young children, and the
associated risk of chronic otitis media. Age, family history, and
sex are not modifiable risk factors, and attendance at a child-care
center outside the home is a necessity for many families. However,
more can be done to prevent otitis media.
Pneumococcal capsular polysaccharide is the principal virulence
factor protecting pneumococci against the defense mechanisms of
the host. Capsular polysaccharide is a T-cell–independent antigen
and, consequently, does not elicit antibody responses in young
infants, who lack the mature B lymphocytes necessary for
T-cell–independent antibody-mediated immunity. For a vaccine to be
effective in infants, it must stimulate a T-cell–dependent antibody
response, which is present soon after birth. The 23-valent pneumococcal
polysaccharide vaccines that have been available since 1977 elicit a
T-cell–independent response and thus do not protect young children,
nor do they reduce the nasopharyngeal carriage of pneumococcus.
The conjugation of capsular polysaccharides to proteins alters
the properties of the antigen complex and converts the antibody response
from T-cell–independent to T-cell–dependent. The experience with H. influenzae type b conjugate vaccines
shows how effective this type of vaccine can be in young children. Several
pneumococcal conjugate vaccines are in development, and they vary
with respect to the serotypes they contain, the carrier proteins
they use, and their conjugation chemistry.29
One such conjugate vaccine, the pneumococcal–CRM197 conjugate vaccine,
has been approved by the FDA for routine use in infants and toddlers
to prevent invasive pneumococcal disease. This conjugate vaccine is
a mixture of six purified pneumococcal capsular polysaccharides and
one capsular oligosaccharide, each coupled to a nontoxic variant of
diphtheria toxin (CRM197). Although no vaccine has been developed to
cover all 90 known pneumococcal serotypes, the 7 serotypes included
in this vaccine — 4, 6B, 9V, 14, 18C, 19F, and 23F — are those that
cause 80 percent of invasive pneumococcal disease in children and
approximately 60 percent of pneumococcal acute otitis media.30,31
These serotypes are also the most resistant to antibiotic therapy,30,31,32
although resistance is emerging among serotypes not covered by
the vaccine.13
Other pneumococcal conjugate vaccines that are in development
include more serotypes and use different carrier proteins for
conjugation and different conjugation chemistry. For example, a
9-valent pneumococcal conjugate vaccine currently under study in
Israel and Gambia contains serotypes 1 and 5 in addition to those
contained in the pneumococcal–CRM197 conjugate vaccine.
When it is given according to a four-dose schedule of
administration at 2, 4, 6, and 12 to 15 months of age, the
pneumococcal–CRM197 conjugate vaccine is more than 97 percent
effective in preventing invasive pneumococcal disease in healthy
infants who have received some or all of these doses of vaccine.11
Unlike the 23-valent pneumococcal polysaccharide vaccines, the
pneumococcal–CRM197 conjugate vaccine can induce immunity in the
population that is at highest risk for disease. Recently, the
Advisory Committee on Immunization Practices and the American
Academy of Pediatrics issued recommendations advocating the routine
administration of this vaccine to all children 23 months of age or
younger and to children between 24 and 59 months of age who are at
high risk for pneumococcal infection (i.e., those with conditions
causing immunocompromise and certain underlying medical conditions).33,34
The recommendations also indicate that, when possible, clinicians should
consider vaccinating all other children 24 to 59 months of age, with
priority given to those who are at moderate risk for pneumococcal
infection, including Alaskan Native and American Indian children,
those of African-American descent, and children attending child-care
centers outside the home.33
In addition to their immunogenicity and efficacy in preventing
invasive disease, pneumococcal conjugate vaccines have been shown
to reduce nasopharyngeal carriage of pneumococci35,36,37
and to reduce the frequency of acute otitis media.11,38,39
In a randomized, double-blind clinical trial conducted at 23
Northern California Kaiser Permanente medical centers enrolling more
than 37,000 children,11
the efficacy of the pneumococcal–CRM197 conjugate vaccine in
preventing otitis media was assessed as a secondary outcome.
Vaccination reduced the number of episodes of acute otitis media by
7.0 percent and the number of visits to physicians' offices for
otitis media by 8.9 percent. The efficacy rate was higher — 22.8
percent — when it was measured in terms of the prevention of
frequent otitis media, defined as the occurrence of five episodes of
acute otitis media during a six-month period or six episodes during
the course of one year (Table 1).
Children who received the vaccine were 20.1 percent less likely than
controls to require tympanostomy tubes for recurrent acute otitis
media or chronic otitis media with effusion. These results are
consistent with a study that demonstrated significantly higher
concentrations of anticapsular antibody in otitis-prone children
after the administration of the pneumococcal–CRM197 conjugate
vaccine than after the administration of a 23-valent pneumococcal
polysaccharide vaccine.40
|
The ability of pneumococcal conjugate vaccines to protect children against
acute otitis media was more thoroughly evaluated in the Finnish
Otitis Media Study.38,39
A total of 2497 children were randomly assigned to receive either
the pneumococcal–CRM197 conjugate vaccine, another 7-valent
pneumococcal conjugate vaccine (pneumococcal polysaccharides
conjugated to meningococcal outer membrane protein complex [OMPC]),
or a control (hepatitis B) vaccine at 2, 4, 6, and 12 months of age.
Children were followed to 24 months of age. In the comparison
between the pneumococcal–CRM197 group and the control group, 2596
episodes of acute otitis media were diagnosed. The overall incidence
of acute otitis media was 1.16 episodes per person-year in the
pneumococcal–CRM197 vaccine group and 1.24 episodes per person-year
in the control group. Samples of middle-ear fluid were obtained for
culture from children during 93 percent of all episodes of acute
otitis media; 271 episodes of culture-confirmed pneumococcal acute
otitis media occurred in the pneumococcal-vaccine group, and 414
episodes occurred in the control group — a 34 percent reduction in
the incidence of pneumococcal acute otitis media as a result of
vaccination. The efficacy rates were 57 percent against episodes due
to the serotypes contained in the vaccine and 51 percent against
cross-reactive serotypes (6A, 9N, 18B, 19A, and 23A).
In the comparison between the pneumococcal–OMPC vaccine group
and the control group in the Finnish Otitis Media Study, there were
110 episodes of acute otitis media attributed to the serotypes
contained in the vaccine in the pneumococcal–OMPC vaccine group and
250 such episodes in the control group — a reduction of 56 percent
(95 percent confidence interval, 44 to 66 percent) with vaccination.39
The overall incidence of acute otitis media did not differ between
the groups. For the booster dose at 12 months, the last 187 children
in the pneumococcal–OMPC conjugate-vaccine group received the
23-valent pneumococcal polysaccharide vaccine, and the others in
that group received pneumococcal–OMPC vaccine. Vaccine efficacy
after the booster dose was nearly identical in these two subgroups
(62 percent and 61 percent, respectively), suggesting that the
pneumococcal–OMPC conjugate vaccine effectively primes the child's
system for a booster dose of either conjugate or polysaccharide.
These results are interesting in the light of the fact that, when
given to infants at 2, 4, 6, and 15 months of age, the pneumococcal–OMPC
vaccine apparently has lower immunogenicity41
than the pneumococcal–CRM197 conjugate vaccine.42
A recent analysis of 500 isolates obtained from the middle-ear
fluid of patients with pneumococcal acute otitis media (90 percent of
whom were children) found that 67 percent of all isolates were
covered by the 7-valent pneumococcal conjugate vaccines, and 67
percent and 77 percent, respectively, would be covered by 9-valent
and 11-valent pneumococcal conjugate vaccines that are now in
development.43
Moreover, 97 percent of antibiotic-resistant strains were covered by
each of the vaccine formulations, with no significant differences
among the three vaccines. The fact that there was a reduction in
pneumococcal carriage and in the prevalence of drug-resistant S. pneumoniae involving the serotypes
contained in the vaccine suggests that there is potential for herd
immunity, in which the spread of the serotypes that are most
commonly associated with disease and antibiotic resistance would be
reduced.36
Expectations of Vaccines and Future Vaccine
Strategies
Although the pneumococcal–CRM197 conjugate vaccine has a
small effect on individual children, it is likely to have a large
effect on the prevalence of pneumococcal disease at the population
level; it is also likely to cause a considerable reduction in the
absolute number of episodes of otitis media, since otitis media is
such a common disease.44
It has been projected that vaccination with pneumococcal–CRM197
conjugate could prevent more than 12,000 cases of meningitis and
bacteremia, 53,000 cases of pneumonia, 116 pneumococcal-related deaths,
and 1 million episodes of otitis media per vaccinated birth cohort
in the United States.32
It has also been estimated that the effect on otitis media would
account for 60 percent of the cost savings expected from the
prevention of pneumococcal disease through a vaccination program.32
One problem is that pediatricians and parents expect that
universally administered vaccines will virtually eradicate the
disease they target. Clearly, the pneumococcal–CRM197 conjugate
vaccine is highly effective in reducing the prevalence of invasive
pneumococcal disease. However, its rates of efficacy against acute
otitis media are substantially lower than the efficacy rates of
vaccines for other vaccine-preventable diseases. Thus, the
expectations of physicians and parents regarding the vaccine are not
likely to be met solely on the basis of the prevention of otitis
media.
The current strategy for the use of the pneumococcal–CRM197 conjugate
vaccine relies on vaccination to eliminate most disease-causing pneumococci,
including those that are most resistant to antibiotic therapy. Two
possible future scenarios could delay progress. The serotypes
contained in the vaccine are capable of capsular transformation into
other serotypes that can cause disease. Serotype 14 variants of the
Spanish clone of serotype 9V and serotype 19F variants of the
multidrug-resistant Spanish clone of serotype 23F have arisen
through large recombinational replacements of the genetic locus
responsible for biosynthesis of the capsule.45,46
The pneumococcal–CRM197 conjugate vaccine is recommended for
routine use in infants and toddlers — persons in whom pneumococcal
carriage is significantly more common than it is in adults, which
might increase the likelihood of capsular transformation.
The vaccine covers only 7 of the 90 known disease-causing
serotypes of pneumococcus, and its widespread use might therefore
allow some of the 83 other serotypes to become predominant
pathogens. In the Finnish Otitis Media Study, there was a 33 percent
increase among the children vaccinated with the pneumococcal–CRM197
conjugate in the incidence of pneumococcal otitis media caused by
serotypes not included in the vaccine.38
Although the use of the 23-valent vaccine has not been associated
with an apparent increase in disease caused by serotypes not
included in that vaccine, it has been used primarily in adults, who
have substantially lower rates of pneumococcal carriage.
In the future, pneumococcal-protein vaccines should offer an alternative
approach to the prevention of pneumococcal disease and the reduction
of carriage. Several proteins that are virulence factors in
pneumococcus have been identified. One, pneumococcal surface protein
A (PspA), is effective against otitis media in rats, and a mixture
of two proteins (PspA and pneumococcal surface adhesion A protein
[PsaA]) protects against nasal carriage of pneumococci in mice.47
A third protein, pneumolysin, together with PspA, elicits protection
against invasive disease. Including these proteins in
polysaccharide–protein conjugate vaccines might enhance the
vaccines' efficacy against otitis media while providing coverage
against serotypes not included in the vaccines.
Viral vaccines provide another approach to the prevention of otitis
media, since respiratory virus infection contributes importantly to
the pathogenesis of acute otitis media.48
At present, influenzavirus vaccine is the only commercially
available vaccine for the control of respiratory virus infections.
Results of three randomized trials of influenzavirus vaccine
indicate that the prevention of viral infection, which usually
precedes acute otitis media, is an effective way to prevent the
development of acute otitis media. In a Finnish study, the
administration of inactivated influenzavirus vaccine led to an
incidence of acute otitis media associated with influenza A that was
83 percent lower than that among controls and an overall incidence
of acute otitis media that was 36 percent lower than that among
controls.49
Similar results were found in a study in the United States, in
which there were 32 percent fewer episodes of acute otitis media in
vaccinated children during the influenza season studied than in
controls.50
A live attenuated intranasal influenzavirus vaccine was 93 percent
effective against culture-confirmed influenza, and vaccinated
children had 30 percent fewer episodes of febrile acute otitis
media.51
Respiratory syncytial virus vaccines that are currently being
developed do not seem to induce protection against lower-airway disease
in young infants and children, and they have not been evaluated in
terms of protection against otitis media. However, passively
administered antibody against this virus was effective against both
respiratory syncytial virus lower-airway disease52
and otitis media in a multicenter trial.53
Two attenuated parainfluenza virus vaccines have been found to be
safe and immunogenic in infants, but there are insufficient data on
their ability to protect against respiratory disease.54,55
Currently, routine vaccination of all children with the
pneumococcal–CRM197 conjugate vaccine is our best strategy for
reducing the burden of early-childhood pneumococcal diseases,
including otitis media. Continued surveillance of the distribution
of pneumococcal serotypes and patterns of drug resistance is
necessary and will dictate the development of future vaccine
approaches.
Source Information
From the Department of Pediatrics, University of Minnesota School
of Medicine, Minneapolis.
Address reprint requests to Dr. Giebink at the Department of
Pediatrics, University of Minnesota School of Medicine, MMC-296, 420 Delaware
St. S.E., Minneapolis, MN 55455, or at [log in to unmask].
References
- Freid
VM, Makuc DM, Rooks RN. Ambulatory health care visits by children:
principal diagnosis and place of visit. Vital and health statistics.
Series 13. No. 137. Washington, D.C.: Government Printing Office, 1998.
(DHHS publication no. (PHS) 98-1798.)
- Teele
DW, Klein JO, Rosner B. Epidemiology of otitis media during the first
seven years of life in children in greater Boston: a prospective, cohort
study. J Infect Dis 1989;160:83-94.
[Medline]
- Gates
GA. Cost-effectiveness considerations in otitis media treatment.
Otolaryngol Head Neck Surg 1996;114:525-530.
[Medline]
- Prevention
of pneumococcal disease: recommendations of the Advisory Committee on
Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 1997;46:1-24.
- Dowell
SF, Butler JC, Giebink GS, et al. Acute otitis media: management and
surveillance in an era of pneumococcal resistance -- a report from the
Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr
Infect Dis J 1999;18:1-9. [Erratum, Pediatr Infect Dis J 1999;18:341.]
[Medline]
- Block
SL. Causative pathogens, antibiotic resistance and therapeutic
considerations in acute otitis media. Pediatr Infect Dis J 1997;16:449-456.
[Medline]
- Pneumococcal
vaccines: WHO position paper. Wkly Epidemiol Rec 1999;74:177-183.
[Medline]
- Baraff
LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a
meta-analysis. Pediatr Infect Dis J 1993;12:389-394.
[Medline]
- Juven T,
Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in
254 hospitalized children. Pediatr Infect Dis J 2000;19:293-298.
[Medline]
- Gendrel
D, Raymond J, Moulin F, et al. Etiology and response to antibiotic therapy
of community-acquired pneumonia in French children. Eur J Clin Microbiol
Infect Dis 1997;16:388-391.
[Medline]
- Black S,
Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of
heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis
J 2000;19:187-195.
[Medline]
- Jacobs
MR, Bajaksouzian S, Zilles A, Lin G, Pankuch GA, Appelbaum PC.
Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to
10 oral antimicrobial agents based on pharmacodynamic parameters: 1997
U.S. surveillance study. Antimicrob Agents Chemother 1999;43:1901-1908.
[Abstract/Full Text]
- Whitney
CG, Farley MM, Hadler J, et al. Increasing prevalence of
multidrug-resistant Streptococcus pneumoniae in the United States. N Engl
J Med 2000;343:1917-1924.
[Abstract/Full Text]
- Kaleida
PH, Casselbrant ML, Rockette HE, et al. Amoxicillin or myringotomy or both
for acute otitis media: results of a randomized clinical trial. Pediatrics
1991;87:466-474.
[Abstract]
- Breiman
RF, Butler JC, Tenover FC, Elliott JA, Facklam RR. Emergence of
drug-resistant pneumococcal infections in the United States. JAMA
1994;271:1831-1835.
[Medline]
- Butler
JC, Hofmann J, Cetron MS, Elliott JA, Facklam RR, Breiman RF. The
continued emergence of drug-resistant Streptococcus pneumoniae in the
United States: an update from the Centers for Disease Control and
Prevention's Pneumococcal Sentinel Surveillance System. J Infect Dis 1996;174:986-993.
[Medline]
- Daly KA,
Brown JE, Lindgren BR, Meland MH, Le CT, Giebink GS. Epidemiology of
otitis media onset by six months of age. Pediatrics 1999;103:1158-1166.
[Abstract/Full Text]
- Levine
OS, Farley M, Harrison LH, Lefkowitz L, McGeer A, Schwartz B. Risk factors
for invasive pneumococcal disease in children: a population-based
case-control study in North America. Pediatrics 1999;103:656-656.abstract
- Paradise
JL, Rockette HE, Colborn DK, et al. Otitis media in 2253 Pittsburgh-area
infants: prevalence and risk factors during the first two years of life.
Pediatrics 1997;99:318-333.
[Abstract/Full Text]
- Block
SL, Harrison CJ, Hedrick JA, et al. Penicillin-resistant Streptococcus
pneumoniae in acute otitis media: risk factors, susceptibility patterns
and antimicrobial management. Pediatr Infect Dis J 1995;14:751-759.
[Medline]
- Jacobs
MR, Dagan R, Appelbaum PC, Burch DJ. Prevalence of antimicrobial-resistant
pathogens in middle ear fluid: multinational study of 917 children with
acute otitis media. Antimicrob Agents Chemother 1998;42:589-595.
[Abstract/Full Text]
- Zenni
MK, Cheatham SH, Thompson JM, et al. Streptococcus pneumoniae colonization
in the young child: association with otitis media and resistance to
penicillin. J Pediatr 1995;127:533-537.
[Medline]
- Boken
DJ, Chartrand SA, Goering RV, Kruger R, Harrison CJ. Colonization with
penicillin-resistant Streptococcus pneumoniae in a child-care center.
Pediatr Infect Dis J 1995;14:879-884.
[Medline]
- Duchin
JS, Breiman RF, Diamond A, et al. High prevalence of multidrug-resistant
Streptococcus pneumoniae among children in a rural Kentucky community.
Pediatr Infect Dis J 1995;14:745-750.
[Medline]
- Boken
DJ, Chartrand SA, Moland ES, Goering RV. Colonization with penicillin-nonsusceptible
Streptococcus pneumoniae in urban and rural child-care centers. Pediatr
Infect Dis J 1996;15:667-672.
[Medline]
- Dagan R,
Leibovitz E, Greenberg D, Yagupsky P, Fliss DM, Leiberman A. Dynamics of
pneumococcal nasopharyngeal colonization during the first days of
antibiotic treatment in pediatric patients. Pediatr Infect Dis J
1998;17:880-885.
[Medline]
- Dowell
SF, Schwartz B. Resistant pneumococci: protecting patients through
judicious use of antibiotics. Am Fam Physician 1997;55:1647-1648.
[Medline]
- Berman
S. Otitis media in children. N Engl J Med 1995;332:1560-1565.
[Full Text]
- Klein
DL. Pneumococcal conjugate vaccines: review and update. Microb Drug Resist
1995;1:49-58.
[Medline]
- Hausdorff
WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific
pneumococcal serogroups to different disease manifestations: implications
for conjugate vaccine formulation and use. Clin Infect Dis 2000;30:122-140.
[Medline]
- Hausdorff
WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause
the most invasive disease: implications for conjugate vaccine formulation
and use. Clin Infect Dis 2000;30:100-121.
[Medline]
- Lieu TA,
Ray GT, Black SB, et al. Projected cost-effectiveness of pneumococcal
conjugate vaccination of healthy infants and young children. JAMA
2000;283:1460-1468.
[Medline]
- Preventing
pneumococcal disease among infants and young children: recommendations of
the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal
Wkly Rep 2000;49:1-35.
- American
Academy of Pediatrics, Committee on Infectious Diseases. Policy statement:
recommendations for the prevention of pneumococcal infections, including
the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal
polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics
2000;106:362-366.
[Abstract/Full Text]
- Dagan R,
Melamed R, Muallem M, et al. Reduction of nasopharyngeal carriage of
pneumococci during the second year of life by a heptavalent conjugate
pneumococcal vaccine. J Infect Dis 1996;174:1271-1278.
[Medline]
- Dagan R,
Fraser D. Conjugate pneumococcal vaccine and antibiotic-resistant
Streptococcus pneumoniae: herd immunity and reduction of otitis morbidity.
Pediatr Infect Dis J 2000;19:Suppl:S79-S87.
[Medline]
- Mbelle
N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and
impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate
vaccine. J Infect Dis 1999;180:1171-1176.
[Medline]
- Eskola
J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine
against acute otitis media. N Engl J Med 2001;344:403-409.
[Abstract/Full Text]
- Kilpi T,
Palmu A, Leinonen M, et al. Efficacy of a seven-valent pneumococcal
conjugate vaccine (PncOMPC) against serotype-specific acute otitis media
caused by Streptococcus pneumoniae (Pnc). In: Program and abstracts of the
40th Interscience Conference on Antimicrobial Agents and Chemotherapy,
Toronto, September 17–20, 2000. Washington, D.C.: American Society for
Microbiology, 2000:245. abstract.
- Barnett
ED, Pelton SI, Cabral HJ, et al. Immune response to pneumococcal conjugate
and polysaccharide vaccines in otitis-prone and otitis-free children. Clin
Infect Dis 1999;29:191-192.
[Medline]
- Miernyk
KM, Parkinson AJ, Rudolph KM, et al. Immunogenicity of a heptavalent pneumococcal
conjugate vaccine in Apache and Navajo Indian, Alaska Native, and
non-Native American children aged <2 years. Clin Infect Dis
2000;31:34-41.
[Medline]
- Rennels
MB, Edwards KM, Keyserling HL, et al. Safety and immunogenicity of
heptavalent pneumococcal vaccine conjugated to CRM197 in United
States infants. Pediatrics 1998;101:604-611.
[Abstract/Full Text]
- Joloba
ML, Jacobs MR, Windau A, Bajaksouzian S, Appelbaum PC, Hausdorff WP.
Susceptibility of 7-valent pneumococcal conjugate vaccine serotypes versus
non-vaccine serotypes against otitis media isolates of Streptococcus
pneumoniae. In: Program and abstracts of the 40th Interscience Conference
on Antimicrobial Agents and Chemotherapy, Toronto, September 17–20, 2000.
Washington, D.C.: American Society for Microbiology, 2000:235. abstract.
- Kilpi T,
Eskola J, Mäkelä PH. A pneumococcal conjugate vaccine and acute otitis
media. N Engl J Med 2001;344:1720-1720.
- Coffey
TJ, Daniels M, Enright MC, Spratt BG. Serotype 14 variants of the Spanish
penicillin-resistant serotype 9V clone of Streptococcus pneumoniae arose by
large recombinational replacements of the cpsA-pbp1a region. Microbiology
1999;145:2023-2031.
[Abstract]
- Coffey
TJ, Enright MC, Daniels M, et al. Recombinational exchanges at the
capsular polysaccharide biosynthetic locus lead to frequent serotype
changes among natural isolates of Streptococcus pneumoniae. Mol Microbiol
1998;27:73-83.
[Medline]
- Briles
DE, Hollingshead SK, Nabors GS, Paton JC, Brooks-Walter A. The potential
for using protein vaccines to protect against otitis media caused by
Streptococcus pneumoniae. Vaccine 2000;19:Suppl 1:S87-S95.
[Medline]
- Pitkaranta
A, Virolainen A, Jero J, Arruda E, Hayden FG. Detection of rhinovirus,
respiratory syncytial virus, and coronavirus infections in acute otitis media
by reverse transcriptase polymerase chain reaction. Pediatrics
1998;102:291-295.
[Abstract/Full Text]
- Heikkinen
T, Ruuskanen O, Waris M, Ziegler T, Arola M, Halonen P. Influenza
vaccination in the prevention of acute otitis media in children. Am J Dis
Child 1991;145:445-448.
[Medline]
- Clements
DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the
incidence of otitis media in 6- to 30-month-old children in day care. Arch
Pediatr Adolesc Med 1995;149:1113-1117.
[Medline]
- Belshe
RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated,
cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N
Engl J Med 1998;338:1405-1412.
[Abstract/Full Text]
- The
PREVENT Study Group. Reduction of respiratory syncytial virus
hospitalization among premature infants and infants with bronchopulmonary
dysplasia using respiratory syncytial virus immune globulin prophylaxis.
Pediatrics 1997;99:93-99.
[Abstract/Full Text]
- Simoes
EA, Groothuis JR, Tristram DA, et al. Respiratory syncytial virus-enriched
globulin for the prevention of acute otitis media in high risk children. J
Pediatr 1996;129:214-219.
[Medline]
- Karron
RA, Wright PF, Newman FK, et al. A live human parainfluenza type 3 virus
vaccine is attenuated and immunogenic in healthy infants and children. J
Infect Dis 1995;172:1445-1450.
[Medline]
- Karron
RA, Wright PF, Hall SL, et al. A live attenuated bovine parainfluenza
virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically
stable in infants and children. J Infect Dis 1995;171:1107-1114.
[Medline]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.