|
|
A Randomized Trial of the Angiotensin-Receptor
Blocker Valsartan in Chronic Heart Failure
Jay N. Cohn, M.D., Gianni Tognoni, M.D., for the Valsartan
Heart Failure Trial Investigators
ABSTRACT
Background Actions of
angiotensin II may contribute to the progression of heart failure
despite treatment with currently recommended drugs. We therefore
evaluated the long-term effects of the addition of the
angiotensin-receptor blocker valsartan to standard therapy for heart
failure.
Methods A total of
5010 patients with heart failure of New York Heart Association
(NYHA) class II, III, or IV were randomly assigned to receive 160 mg
of valsartan or placebo twice daily. The primary outcomes were
mortality and the combined end point of mortality and morbidity,
defined as the incidence of cardiac arrest with resuscitation,
hospitalization for heart failure, or receipt of intravenous
inotropic or vasodilator therapy for at least four hours.
Results Overall
mortality was similar in the two groups. The incidence of the
combined end point, however, was 13.2 percent lower with valsartan
than with placebo (relative risk, 0.87; 97.5 percent confidence
interval, 0.77 to 0.97; P=0.009), predominantly because of a lower
number of patients hospitalized for heart failure: 455 (18.2
percent) in the placebo group and 346 (13.8 percent) in the
valsartan group (P<0.001). Treatment with valsartan also resulted
in significant improvements in NYHA class, ejection fraction, signs
and symptoms of heart failure, and quality of life as compared with
placebo (P<0.01). In a post hoc analysis of the combined end
point and mortality in subgroups defined according to base-line
treatment with angiotensin-converting–enzyme (ACE) inhibitors or
beta-blockers, valsartan had a favorable effect in patients
receiving neither or one of these types of drugs but an adverse
effect in patients receiving both types of drugs.
Conclusions Valsartan
significantly reduces the combined end point of mortality and
morbidity and improves clinical signs and symptoms in patients with
heart failure, when added to prescribed therapy. However, the post
hoc observation of an adverse effect on mortality and morbidity in
the subgroup receiving valsartan, an ACE inhibitor, and a
beta-blocker raises concern about the potential safety of this
specific combination.
Pharmacotherapy for heart failure has
advanced considerably in recent years as clinical trials have
demonstrated favorable long-term effects of
angiotensin-converting–enzyme (ACE) inhibitors1,2,3 and
beta-blockers4,5,6 on
morbidity and mortality. Despite the use of these potent drugs,
heart failure remains the leading reason for hospitalization in the
Medicare population,7
mortality among patients with heart failure is high, and the quality
of life is low.
Angiotensin II, a potent vasoconstrictor and growth-stimulating
hormone, may contribute to the impairment of left ventricular function
and the progression of heart failure through increased impedance of
left ventricular emptying,8
adverse long-term structural effects on the heart and vasculature,9 and
potentially deleterious activation of other neurohormonal agonists,
including norepinephrine, aldosterone, and endothelin.10
Since previous studies have shown that physiologically active levels
of angiotensin II persisted despite long-term therapy with an ACE
inhibitor,11,12
we undertook a study to determine whether the angiotensin-receptor
blocker valsartan could further reduce morbidity and mortality among
patients who were already receiving the pharmacologic therapy that
was considered optimal by their physicians. Descriptions of the
rationale for and design of this trial have been published elsewhere.13
Methods
Study Design
The Valsartan Heart Failure Trial (Val-HeFT) was a randomized,
placebo-controlled, double-blind, parallel-group trial. Patients at
302 centers in 16 countries gave written informed consent for
participation in the trial, which was approved by the institutional review
board at each center. The investigation conformed to the principles
of the Declaration of Helsinki. Site monitoring, data collection,
and data analysis were performed by Novartis Pharmaceuticals. An
independent end-points committee adjudicated all reports of primary
end points. An independent data and safety monitoring board reviewed
biannual interim analyses. The manuscript was prepared by the authors
and reviewed by the steering committee and the sponsor.
Eligibility
Men and women 18 years old or older with a history and clinical
findings of heart failure for at least three months before screening
were eligible. Patients had heart failure of New York Heart Association
(NYHA) class II, III, or IV and were clinically stable. To be
eligible, they had to have been receiving for at least two weeks a
fixed-dose drug regimen that could include ACE inhibitors,
diuretics, digoxin, and beta-blockers. In addition, they had to have
documented left ventricular dysfunction with an ejection fraction of
less than 40 percent and left ventricular dilatation with an
echocardiographically measured short-axis internal dimension at end
diastole greater than 2.9 cm per square meter of body-surface area.
Echocardiograms were analyzed locally after the technical and reader
quality at each center had been validated by one of three core
laboratories (in Los Angeles; Milan, Italy; or Stockholm, Sweden)
that also monitored quality control during the study. Criteria for
exclusion have been published previously.13
Placebo Run-in Period
Patients were assessed for two to four weeks to confirm their
eligibility, clinical stability, and compliance while taking placebo
in a single-blind fashion twice daily. Base-line evaluations included
laboratory tests for hematologic variables and blood chemistry;
urinalysis; echocardiography; 12-lead electrocardiography; and chest
radiography. Quality of life was assessed with the Minnesota Living
with Heart Failure questionnaire, which was administered to 60
percent of patients — that is, those in the United States, the
United Kingdom, Australia, and Italy.
Randomization and Dose Adjustment
Eligible patients, stratified according to whether or not they
were receiving a beta-blocker as background therapy, were randomly assigned
to receive oral valsartan or matching placebo. Stratification was
performed to ensure the equal distribution of patients receiving these
drugs in the two groups. Randomization occurred after the base-line
eligibility data were verified by the coordinating centers in
Minneapolis and Milan. Valsartan was initiated at a dose of 40 mg
twice daily, and the dose was doubled every two weeks until a target
dose of 160 mg twice daily was reached. Placebo doses were similarly
adjusted. The criteria for increasing the dose included a systolic
blood pressure of 90 mm Hg or higher while the patient was standing,
the absence of symptoms of hypotension, and a serum creatinine
concentration of less than 2.0 mg per deciliter (177 µmol per liter)
or no more than 50 percent higher than the base-line concentration.
Patients returned for follow-up visits at two, four, and six months
and every three months thereafter.
Outcome Measures
The study was designed with two primary end points: mortality
and the combined end point of mortality and morbidity, which was
defined as cardiac arrest with resuscitation, hospitalization for
heart failure, or administration of intravenous inotropic or
vasodilator drugs for four hours or more without hospitalization. Secondary
cardiovascular outcomes included the changes from base line to the
last available observation after treatment had begun in ejection
fraction, NYHA functional class, quality-of-life scores, and signs
and symptoms of heart failure.
Statistical Analysis
Statistical analyses were performed at an overall significance
level of 0.05, adjusted for the two primary end points. Each primary
end point was tested at a two-sided significance level of 0.02532,
on the basis of the Dunn–Sidak inequality: ![]() '=1–(1–
'=1–(1–![]() )1/2. The
significance level for the analysis of the time to death was further
adjusted for five biannual interim analyses according to the
O'Brien–Fleming alpha-spending function. Therefore, the final
analysis for the time to death was performed at a two-sided
significance level of 0.02.
)1/2. The
significance level for the analysis of the time to death was further
adjusted for five biannual interim analyses according to the
O'Brien–Fleming alpha-spending function. Therefore, the final
analysis for the time to death was performed at a two-sided
significance level of 0.02.
The calculation of sample size was based on the time-to-death
end point. The number of deaths that would be required to detect, with
90 percent power, a 20 percent difference between the death rate
with valsartan and that with placebo (estimated at 12 percent per
year) was calculated to be 906. We planned to enroll 2500 patients
per treatment group.
Comparisons of the primary end points between treatment groups
were performed by means of a log-rank test. To estimate the size
of the effect, we used a Cox regression model with prespecified base-line
covariates, including NYHA class, ejection fraction (above or below
the median), cause of heart failure (ischemic or nonischemic), age
(younger than 65 years or 65 years old or older), ACE inhibitor use
or nonuse, and beta-blocker use or nonuse. Confidence intervals of
98 percent and 97.5 percent were calculated for mortality and the
combined end point of mortality and morbidity, respectively. To
estimate the size of the effect on the secondary end points and in
subgroups, relative risks with 95 percent confidence intervals were
calculated with the use of the Cox regression model.
Results
Of the 5010 patients who underwent randomization, 2511 were assigned
to receive valsartan and 2499 to receive placebo, all with
background therapy for heart failure. There were no clinically relevant
differences in the base-line characteristics of the two groups (Table 1). A
description of the base-line demographic characteristics of this
diverse population has been published previously.14
At the time of randomization, 93 percent of the patients were being
treated with ACE inhibitors. The average daily doses were 17 mg of
enalapril, 19 mg of lisinopril, 80 mg of captopril, 6 mg of ramipril,
and 23 mg of quinapril. Thirty-five percent of the patients were
receiving beta-blockers (15 percent were receiving carvedilol, 12
percent metoprolol, and 3 percent atenolol), and randomization was
stratified according to their use or nonuse; this percentage
remained stable throughout the study. Only 5 percent of the patients
were treated with spironolactone. The overall mean duration of
follow-up was 23 months (range, 0 to 38).
|
The target dose was achieved in 84 percent of the patients receiving valsartan
(mean dose, 254 mg) and 93 percent of those receiving placebo (mean
equivalent dose, 283 mg). Systolic blood pressure was reduced to a
greater extent with valsartan than placebo: at four months, it was
reduced by a mean (±SD) of 5.2±15.8 mm Hg in the valsartan group, as
compared with 1.2±14.8 mm Hg in the placebo group, and at one year
the reductions were 5.2±16.0 mm Hg and 1.3±15.9 mm Hg, respectively.
The mean heart rate was unchanged.
Primary End Points
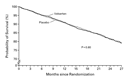
Mortality was similar in the two treatment groups (Figure 1 and
Table 2). The
adjudicated causes of death were also similar in the two treatment
groups (there were 262 sudden deaths from cardiac causes in the
valsartan group and 258 in the placebo group, and there were 118
deaths due to heart failure in the valsartan group and 125 in the
placebo group).
|
|
The combined end point of mortality and morbidity was significantly reduced
among patients receiving valsartan as compared with those receiving
placebo (P=0.009) (Figure
2). The benefit appeared early after randomization and increased
throughout the trial. Among the patients in the valsartan group, 723
(28.8 percent) reached the combined end point, as compared with 801
patients (32.1 percent) in the placebo group — a 13.2 percent
reduction in risk with valsartan (relative risk, 0.87; 97.5 percent
confidence interval, 0.77 to 0.97) (Table 2). The
predominant benefit in terms of the combined end point was a 24
percent reduction in the rate of adjudicated hospitalizations for
worsening heart failure as a first event in those receiving
valsartan (13.8 percent) as compared with those receiving placebo
(18.2 percent) (P<0.001) (Table 2).
|
Secondary End Points
The risk of a hospitalization for heart failure (with censoring
of the data for patients who died) was reduced by 27.5 percent with
valsartan (P<0.001). There were 1189 nonadjudicated hospitalizations
for heart failure in the placebo group and 923 in the valsartan
group (P=0.002). Since hospitalizations for problems other than
heart failure were unaffected, the rate of hospitalizations for any
cause was reduced similarly — by 250 events, from 3106 in the
placebo group to 2856 in the valsartan group (P=0.14). The mean
change in ejection fraction from base line to the last observation
was 4.0 percent in the valsartan group and 3.2 percent in the
placebo group (P=0.001). More patients in the valsartan group than
in the placebo group had improvements in NYHA classification (23.1
percent vs. 20.7 percent) and fewer had worsening (10.1 percent vs.
12.8 percent) (P<0.001). Similarly, dyspnea, fatigue, edema, and
rales were more favorably affected by valsartan than by placebo
(P<0.01). Among the 1504 patients in the valsartan group to whom
the Minnesota Living with Heart Failure questionnaire was
administered, there was little change in scores from base line to
the end point, but among the 1506 such patients in the placebo
group, the mean score worsened by an average of 1.9 (P=0.005 for the
comparison between the treatment groups).
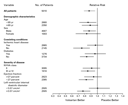
Subgroup Analyses
The beneficial effect of valsartan on the combined
mortality–morbidity end point was generally consistent among the
predefined subgroups of patients. Valsartan improved the outcome in
young and old patients, men and women, those with and without
diabetes or coronary artery disease, those with ejection fractions
or left ventricular dimensions above and below the median, and those
with NYHA class II and class III or IV symptoms (Figure 3). In
the small, heterogeneous black population (which included 344
African-American and South African patients), there was a wide
confidence interval for relative risk of the combined end point with
valsartan that included 1.0 (relative risk, 1.11; 95 percent confidence
interval, 0.77 to 1.61).
|
Background therapy with neurohormonal inhibitors appeared to influence
the response to valsartan (Figure 4). The
patients were divided into four subgroups on the basis of the use or
nonuse of ACE-inhibitor and beta-blocker therapy at base line. The
global test for the interaction between treatment and subgroup among
the four subgroups was statistically significant for mortality (P=0.009)
and the combined end point of mortality and morbidity (P=0.001). In
the three groups receiving neither drug or either ACE inhibitors or
beta-blockers alone, there was a significantly favorable effect of
valsartan on the rate of the combined end point (P=0.003, P=0.002,
and P=0.037, respectively) and a favorable point estimate of the
odds ratio for death. Mortality was significantly reduced in the 226
patients who were treated with neither an ACE inhibitor nor a
beta-blocker (P=0.012). Among those who were receiving both drugs at
base line, valsartan had an adverse effect on mortality (P=0.009)
and was associated with a trend toward an increase in the combined
end point of mortality and morbidity (P=0.10). Among all 366
patients who were not receiving an ACE inhibitor, whether or not a
beta-blocker had been prescribed, there was a significantly lower
risk of the combined end point in the valsartan group than in the
placebo group (relative risk, 0.56; 95 percent confidence interval,
0.39 to 0.81), as well as a lower risk of death (relative risk,
0.67; 95 percent confidence interval, 0.42 to 1.06).
|
Safety
Valsartan therapy was generally well tolerated. Adverse events
leading to the discontinuation of the drug occurred in 249 of the
patients receiving valsartan (9.9 percent) and 181 patients receiving
placebo (7.2 percent) (P<0.001). The adverse events leading to
discontinuation and occurring in more than 1 percent of the patients
in the valsartan group included dizziness (in 1.6 percent of the
patients and 0.4 percent of those in the placebo group; P<0.001),
hypotension (1.3 percent and 0.8 percent, respectively; P=0.124),
and renal impairment (1.1 percent and 0.2 percent, P<0.001).
Overall, the mean change from base line in the blood urea nitrogen
concentration was an increase of 5.9 mg per deciliter (2.1 mmol per
liter) with valsartan and an increase of 3.3 mg per deciliter (9.2
mmol per liter) with placebo (P<0.001). The mean change in the
serum creatinine concentration was an increase of 0.18 mg per
deciliter (15.9 µmol per liter) with valsartan and an increase of
0.10 mg per deciliter (8.8 µmol per liter) with placebo
(P<0.001). The mean change in the serum potassium concentration
was an increase of 0.12 mmol per liter with valsartan and a decrease
of 0.07 mmol per liter with placebo (P<0.001).
Discussion
Our study was designed to assess the efficacy of the
angiotensin-receptor blocker valsartan when added to prescribed
therapy for heart failure. The benefit in terms of morbidity and
mortality was achieved in a population in which 93 percent of
patients were treated with an ACE inhibitor and 35 percent were
treated with a beta-blocker. The outcomes suggest that even with the
use of currently prescribed therapy, angiotensin contributes to
morbidity but not mortality in patients with heart failure. An
unexpected finding emerged, however, from a post hoc analysis of the
data on concomitant therapy. Within the 30 percent of the population
that was being treated with both an ACE inhibitor and a beta-blocker
at base line, there was a significant adverse effect of valsartan on
mortality and a nearly significant adverse effect on morbidity.
Clarification of whether this finding represents a true interaction
or is attributable to chance must await the outcome of ongoing
trials evaluating the combination of an angiotensin-receptor blocker
with an ACE inhibitor and a beta-blocker. Since only 5 percent of
the patients in the trial were receiving spironolactone, an
aldosterone-receptor blocker,15
we cannot assess the efficacy or safety of valsartan when given in
combination with spironolactone.
The protocol was designed with two primary end points and
appropriate statistical adjustment. Although mortality was similar
in the two treatment groups, a significant favorable effect of
valsartan on cardiovascular morbidity was evident, primarily as a
result of a 24 percent reduction in adjudicated (first)
hospitalizations for heart failure and a similar reduction in all
nonadjudicated (subsequent) hospitalizations for heart failure. The
favorable effect was achieved with a target dose of 160 mg twice
daily; this dose was chosen because of its hemodynamic and hormonal
effects, which were documented in a pilot study involving patients who
were receiving ACE-inhibitor therapy.16
The dose was well tolerated; most patients achieved the target dose,
and side effects were only slightly more prevalent than in the
placebo group.
This study differed from previous trials of angiotensin-receptor
blockers in heart failure, such as the Losartan Heart Failure Survival
Study17
and the Randomized Evaluation of Strategies for Left Ventricular
Dysfunction,18
in terms of the high dose we used, our large sample size, and the
use of valsartan as a balanced, placebo-controlled add-on to
background therapy.
The reduction in cardiovascular morbidity has relevance for the
economic burden of heart failure on the health care system. In
addition, the moderate but statistically significant benefit in
terms of the secondary end points — NYHA class, quality of life, signs
and symptoms of heart failure, and left ventricular ejection
fraction — is consistent with an overall incremental benefit of
valsartan for patients with heart failure who are receiving medical
therapy.
The negative effect of angiotensin II on heart failure could be
mediated through a vasoconstrictor-induced increase in blood pressure
or a direct effect on cardiac and vascular tissues. Since systolic
blood pressure was an average of 5 mm Hg lower in patients who were
randomly assigned to receive valsartan than in those assigned to
receive placebo, a hemodynamic mechanism may account, at least in
part, for the observed benefit. Nonetheless, the growth-promoting
and apoptotic effects of angiotensin II have been well demonstrated9,19
and may contribute to the structural remodeling that promotes the
progression of heart failure.20,21,22,23,24
A long-term increase in the ejection fraction has been identified as
a marker of regression of left ventricular remodeling that is
manifested as reduced chamber volume.25,26
This structural effect has been associated with an improvement in
survival.27,28
In our study, the increase in the ejection fraction was more moderate
than in previous trials of ACE inhibitors and beta-blockers and was
not associated with reduced mortality. The absence of a more robust
effect may be related to the effectiveness of the other therapy
received by the patients (annual mortality in the placebo group was
9 percent, rather than the predicted 12 percent).
Subgroup analysis is used in large-scale trials to confirm the
generalizability of the findings or, if inconsistencies are observed,
to generate hypotheses about subgroup responses to be tested in
subsequent studies. In our study, subgroups defined on the basis of
demographic characteristics or base-line clinical characteristics
generally had responses that were similar to those in the study
population as a whole. Background neurohormonal-inhibitor therapy,
however, appeared to influence the outcome. Since this background
therapy was not controlled and patients were only partially
stratified according to its presence or absence at randomization
(according to the use of beta-blockers but not ACE inhibitors), the
data generated by this analysis must be interpreted with caution.
Nonetheless, in the small subgroup of patients (7 percent) who were
not being treated with an ACE inhibitor, there was a 44.0 percent
reduction in the combined end point of mortality and morbidity and a
33.1 percent reduction in mortality.
The point estimate of the odds ratio favored valsartan in all
subgroups except the subgroup of patients who were being treated with
both an ACE inhibitor and a beta-blocker at base line. As previously
noted, the apparent adverse effect of valsartan in this subgroup
leads to the hypothesis that the extensive blockade of multiple
neurohormonal systems in patients with heart failure could be
deleterious. Recent clinical-trial experience with moxonidine,29
endothelin-receptor antagonists, and cytokine inhibitors30
is consistent with this hypothesis. Several trials involving substantial
numbers of patients who are receiving these three classes of
neurohormonal inhibitors are ongoing and can be expected to provide
additional data relevant to this safety concern.
Although current guidelines recommend ACE inhibitors and
beta-blockers as standard therapy for heart failure because of their
demonstrated benefit in terms of mortality, only one third of the
patients enrolled in our study were receiving both classes of drugs.
Furthermore, patients who were already being treated with angiotensin-receptor
blockers, which are widely prescribed for patients who are intolerant
of ACE inhibitors, were excluded from the study. Improved compliance
with the guidelines may reduce the number of inadequately treated patients.
Nonetheless, the benefit of valsartan in terms of the combined end
point of mortality and morbidity that was found in all subgroups
except that receiving both ACE inhibitors and beta-blockers suggests
that the drug could have a role in the management of the syndrome.
Supported by a grant from Novartis Pharma, Basel, Switzerland.
Drs. Cohn and Tognoni have had research support and consultation arrangements
with Novartis Pharmaceuticals, the sponsor of this study.
Presented in part at the American Heart Association meeting, New
Orleans, November 12–15, 2000.
* The investigators are listed in the
Appendix.
Source Information
From the Cardiovascular Division, Department of Medicine,
University of Minnesota Medical School, Minneapolis (J.N.C.); and the Mario
Negri Institute, Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto
Miocardico, Milan, Italy (G.T.).
Address reprint requests to Dr. Cohn at the Cardiovascular
Division, Mayo Mail Code 508, University of Minnesota Medical School, 420
Delaware St., SE, Minneapolis, MN 55455.
References
- The
SOLVD Investigators. Effect of enalapril on survival in patients with
reduced left ventricular ejection fractions and congestive heart failure.
N Engl J Med 1991;325:293-302.
[Abstract]
- The
CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe
congestive heart failure: results of the Cooperative North Scandinavian
Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429-1435.
[Abstract]
- Cohn JN,
Johnson G, Ziesche S, et al. A comparison of enalapril with
hydralazine-isosorbide dinitrate in the treatment of chronic congestive
heart failure. N Engl J Med 1991;325:303-310.
[Abstract]
- CIBIS-II
Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study
II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13.
[Medline]
- MERIT-HF
Study Group. Effect of metoprolol CR/XL in chronic heart failure:
Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure
(MERIT-HF). Lancet 1999;353:2001-2007.
[Medline]
- Packer
M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and
mortality in patients with chronic heart failure. N Engl J Med
1996;334:1349-1355.
[Abstract/Full Text]
- Popovic
JR, Kozak LJ. National Hospital Discharge Survey: annual summary, 1998.
Vital and health statistics. Series 13. No. 148. Washington, D.C.:
Government Printing Office, September 2000. (DHHS publication no. (PHS)
2000-1719.)
- Cohn JN.
Vasodilator therapy for heart failure: the influence of impedance on left
ventricular performance. Circulation 1973;48:5-8.
[Medline]
- Dzau VJ.
Tissue renin-angiotensin system in myocardial hypertrophy and failure.
Arch Intern Med 1993;153:937-942.
[Medline]
- Jilma B,
Krejcy K, Dirnberger E, et al. Effects of angiotensin-II infusion at
pressor and subpressor doses on endothelin-1 plasma levels in healthy men.
Life Sci 1997;60:1859-1866.
[Medline]
- Kawamura
M, Imanashi M, Matsushima Y, Ito K, Hiramori K. Circulating angiotensin II
levels under repeated administration of lisinopril in normal subjects.
Clin Exp Pharmacol Physiol 1992;19:547-553.
[Medline]
- Jorde
UP, Ennezat PV, Lisker J, et al. Maximally recommended doses of
angiotensin-converting enzyme (ACE) inhibitors do not completely prevent
ACE-mediated formation of angiotensin II in chronic heart failure.
Circulation 2000;101:844-846.
[Abstract/Full Text]
- Cohn JN,
Tognoni G, Glazer RD, Spormann D, Hester A. Rationale and design of the
Valsartan Heart Failure trial: a large multinational trial to assess the
effects of valsartan, an angiotensin-receptor blocker, on morbidity and
mortality in chronic congestive heart failure. J Card Fail 1999;5:155-160.
[Medline]
- Cohn JN,
Tognoni G, Glazer R, Spormann D. Baseline demographics of the Valsartan
Heart Failure Trial. Eur J Heart Fail 2000;2:439-446.
[Medline]
- Pitt B,
Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and
mortality in patients with severe heart failure. N Engl J Med
1999;341:709-717.
[Abstract/Full Text]
- Baruch
L, Anand I, Cohen IS, Ziesche S, Judd D, Cohn JN. Augmented short- and
long-term hemodynamic and hormonal effects of an angiotensin receptor
blocker added to angiotensin converting enzyme inhibitor therapy in
patients with heart failure. Circulation 1999;99:2658-2664.
[Abstract/Full Text]
- Pitt B,
Poole-Wilson PA, Segal R, et al. Effect of losartan compared with
captopril on mortality in patients with symptomatic heart failure:
randomised trial -- the Losartan Heart Failure Survival Study ELITE II.
Lancet 2000;355:1582-1587.
[Medline]
- McKelvie
RS, Yusuf S, Pericak D, et al. Comparison of candesartan, enalapril, and their
combination in congestive heart failure: Randomized Evaluation of
Strategies for Left Ventricular Dysfunction (RESOLVD) pilot study.
Circulation 1999;100:1056-1064.
[Abstract/Full Text]
- Leri A,
Liu Y, Li B, et al. Up-regulation of AT1 and AT2
receptors in postinfarcted hypertrophied myocytes and stretch-mediated
apoptotic cell death. Am J Pathol 2000;156:1663-1672.
[Abstract/Full Text]
- Cohn JN,
Ferrari R, Sharpe N. Cardiac remodeling -- concepts and clinical
implications: a consensus paper from an international forum on cardiac
remodeling. J Am Coll Cardiol 2000;35:569-582.
[Medline]
- Dostal
DE, Baker KM. Angiotensin II stimulation of left ventricular hypertrophy
in adult rat heart: mediation by the AT1 receptor. Am J
Hypertens 1992;5:276-280.
[Medline]
- Harrap
SB, Dominiczak AF, Fraser R, et al. Plasma angiotensin II, predisposition
to hypertension, and left ventricular size in healthy young adults.
Circulation 1996;93:1148-1154.
[Abstract/Full Text]
- Jacobi
J, Schlaich MP, Delles C, Schobel HP, Schmieder RE. Angiotensin II
stimulates left ventricular hypertrophy in hypertensive patients
independently of blood pressure. Am J Hypertens 1999;12:418-422.
[Medline]
- Crabos
M, Roth M, Hahn AWA, Erne P. Characterization of angiotensin II receptors
in cultured adult rat cardiac fibroblasts: coupling to signaling systems
and gene expression. J Clin Invest 1994;93:2372-2378.
[Medline]
- Wong M,
Johnson G, Shabetai R, et al. Echocardiographic variables as prognostic
indicators and therapeutic monitors in chronic congestive heart failure:
Veterans Affairs cooperative studies V-HeFT I and II. Circulation 1993;87:Suppl
VI:VI-65.
- Francis
GS, Cohn JN. Heart failure: mechanisms of cardiac and vascular dysfunction
and the rationale for pharmacologic intervention. FASEB J 1990;4:3068-3075.
[Abstract]
- Cintron
G, Johnson G, Francis G, Cobb F, Cohn JN. Prognostic significance of
serial changes in left ventricular ejection fraction in patients with
congestive heart failure. Circulation 1993;87:Suppl VI:VI-17.
- Patten
RD, Udelson JE, Konstam MA. Ventricular remodeling and its prevention in
the treatment of heart failure. Curr Opin Cardiol 1998;13:162-167.
[Medline]
- Jones
CG, Cleland JGF. Meeting report -- the LIDO, HOPE, MOXCON, and WASH
studies. Eur J Heart Fail 1999;1:425-431.
[Medline]
- Louis A,
Cleland JGF, Crabbe F, et al. Clinical trials update: CAPRICORN,
COPERNICUS, MIRACLE, STAF, RITZ-2, RECOVER, and RENAISSANCE and cachexia
and cholesterol in heart failure: highlights of the scientific sessions of
the American College of Cardiology, 2001. Eur J Heart Fail 2001;3:381-387.
[Medline]
Appendix
The Valsartan Heart Failure Trial Investigators included the following:
in the United States: Alabama — R. Bourge, D. Calhoun, B. Foley, L.
Lowe, S. Oparil, G. Perry, B. Rayburn, B. Sanders; Arizona — J.E.
Boulet, J. Christensen, P. Fenster, S. Heumiller, R. Lee, P.
McGowan, J. Ohm, R. Siegel, T. Struiksma; California — J.W. Allen,
J. Backman, P. Coleman, D. Costello, T.A. Cox, P. Deedwania, L.
Defensor, G. Dennish, W.A. Edmiston, D. Everitt, S. Fabbri, C.
Faulkner, J. Gilbert, J.I. Gorwit, S. Harte, L.A. Hawkins, J.
Hemphill, J.T. Heywood, B. Jackson, B. Jaski, S. Khan, D. McAdams,
P. Pak, N. Parker, R. Shabetai, H. Shively, J. Sklar, L. Sprinkle,
R. Stein, P. Waack, R. Wadlington, R. Wright, L.G. Yellen; Connecticut
— S. Carolan, I.S. Cohen, M.B. Fowler, M. Martin-O'Brien, A.
Mullin, T. Ramahi, K. Rholfs; District of Columbia — D.J. Diver, N.
Douglas-Kersellious, D. Lee, S. Singh; Florida — J. Anderson, J.
Bauerlein, M. Bayer, G. Cintron, S. Brenner, C. Davenport, S.L.
Duncan, P. Green, T.C. Hilton, H. Karunaratne, A. Kinsella, G.
Lamas, M. Mayor, M. McIvor, M.R. Milunski, M.A. Nocero, J. O'Bryan,
J. Reddinger, D. Samada, C.R. Stastny, M. Taylor, H. Tee, J.L.
Walker, V. Wilson; Georgia — S. Beer, A. Carr, J. Grace, J. Guidot,
S. Holt, K. Taylor, W.R. Taylor, P. Yaun; Illinois — K. Furlong, M.
Johnson, R. Lang, H. Loeb; Indiana — J. Becker, J. Birt, L. Ford;
Iowa — R. Oren, P. Scovall, W. Wickemeyer, N. Young; Kansas —
M. Bowles; Kentucky — K. Doerschuk, S. Wagner; Louisiana — B. Iteld;
Maryland — L. Black, S. Gottlieb, S.O. Gottlieb, N. Greenberg, D.
Lowry; Massachusetts — A. Burger, M. Burger, K. Coleman, M. Criasia,
W. Dec, C. Haggan, T. Meyer, M. Motta, P. Shrewe, J. Smith; Michigan
— P.A. Kaminski, D. Langholtz, A.B. Levine, T.B. Levine, J. Nicklas,
R. Rose; Minnesota — I. Anand, S. Berg, A. Holmstrom, B.
Merkle, L. Miller; Missouri — K. Aggarwal, E. Geltman, M. Gilbert,
B. Lee, C. Hinden, D. Yip; Nevada — A. Cason-Pitcher, R. Croke, A.
Steljes; New Jersey — R. Berkowitz, H.S. Ribner, J. Strobeck, P.
Tabachnik, M.J. Zucker; New Mexico — M. Conway, P. Doherty, R.
Dubroff, S. Justice; New York — M. Applegate, L. Baruch, C.
Buchholz-Varley, J. Cappelli, M.E. Coglianese, J. Corbelli, T.
Costantino, M.A. Goodman, S. Graham, V. Hart, M. Kukin, O. Ocampo,
W. Orlowski, P. Patacsil, N. Schulhoff, P. Stein, R.M. Steingart,
B.H. Sung, M.F. Wilson; North Carolina — R. Bilbro, F. Cobb, G.
Dodson, D. Framm, K. Harshaw-Ellis, M. Higginbotham, B. Kuzil, G.
Weidner; Ohio — D.J. Kereiakes, R. Lengerich, L.L. Wohlford;
Oklahoma — J. Anderson, J. Cook, M. Dickson, R.D. Ensley, S.
Jameson, J. Kalbfleisch, C. Melson-Alsip, D. Simmons; Oregon — R.
Hershberger, L. Keilson; Pennsylvania — M. Amidi, M. Bell, J.
Boehmer, E. Loh, P.J. Mather, S. Rubin, R. Shannon, I. Smith, R.
Weller-Moore, S. Worley; Rhode Island — A. Sadaniantz; South
Carolina — J. Evans, G. Hendrix; Tennessee — L. Chismark, W.G.
Friesen, S. Gubin, L. Howerton, J. Jeanes, F. McGrew, J. Osborn,
K.B. Ramanathan, R. Smith; Texas — N. Baradhi, L.A. Campos, R.
Carney, S.E. El Hafi, R. Gammon, J.D. Jackman, C. Janes, D.
McCarroll, F. Navetta, M. Rotman, A. Smith; Utah — J.J. Perry;
Virginia — J. Bergin, J. Herre, J. O'Brien; Washington — B. Crane,
D. Fishbein, J. Forster, M. Hall; Wisconsin — C.V. Hughes, J.
Hosenpud, R. Siegel, C.R. Vander Ark, P.A. Wiederholt; outside the
United States: Australia — J. Amerena, I. Button, R. Calvert, M.
Croot, P. Garrahy, C. Hall, H. Hankey, D. Hogan, D. Hollens-Riley,
J. Horowitz, L. Howes, B. Jackson, I. Jeffrey, J. Karrasch, S.
Leslie, H. Krum, L. Martin, B. Singh, P. Taverner, A. Thomson, P.
Thompson; Belgium — S. Degré, P. De Vusser, W. Droogné, P. Noyens,
A.Z.J. Palfijn, A. Strijckmans, J. Vanhaecke, W. Van Mieghem, J.
Vanwelden; Czech Republic — R. Cifkova, J. Gregor, J. Simon, J.
Toman, J. Vojacek, J. Widimsky; Denmark — O. Gøtzsche, T. Haghfelt,
P. Hildebrandt, E. Kassis, T. Toftegaard Nielsen; Finland — E.
Engblom, J. Hartikainen, S. Majahalme, M. Niemelä, K. Peuhkurinen,
M. Pietilä, J. Taurio, L.M. Voipio-Pulkki; France — P. Lechat,
M. Baudet, M. Bory, A. Cohen, P. Coumel, M. Dahan, J.M. Davy, Y.M.
Frances, M. Galinier, M. Guediche, G. Jarry, J.P. Ollivier, L.
Slimane, M. Zaouali; Germany — C. Bergmeier, K.O. Bischoff, C.G.
Brilla, J. Cyran, W.G. Daniel, M. Dürsch, E. Fleck, L.
Goedel-Meinen, R. Griebenow, R. Hambrecht, M. Hamel, K.H. Hauff, G.
Haustein, C. Helzer-Arbeiter, D. Hey, T. Hoefs, M. Hofmann, T.
Kleemann, D. Koch, N. Kokott, T. Langenickel, E. Lopez, T. Matthes,
V. Mitrovic, E. Mueser, K.H. Munderloh, S. Philipp, V.
Regitz-Zagrosek, A. Rouwen, R. Rummel, A. Schmidt, J. Senges, K.
Stumpe, H. Topp, C. Weiler, R. Willenbrock, S. Winkler; Hungary — M.
Csanady, I. Edes, K. Farkas, C. Farsang, T. Forster, T. Fulop, A.
Janosi, L. Mezei, M. Nemeth, E. Palkovi, E. Szigeti; Italy — M.C.
Albanese, G. Alunni, G. Ansalone, B. Aloisi, I. Belloni, R.
Belluschi, D. Bertoli, I. Bisceglia, G.M. Boffa, E. Bosi, A. Branzi,
E. Butti, L. Cacciavillani, C. Campana, S. Capomolla, G.
Castiglioni, A. Cavalli, V. Ceci, E. Cerè, V. Cirrincione, F.
Cobelli, G. Corsini, A.L. Cuzzato, F. De Santis, G. Di Pasquale, M.
Farruggio, C. Fresco, G. Ferrari, G. Filorizzo, G. Foti, A.
Fraticelli, F.A. Galati, K. Ghebremarian-Tesfau, F. Giancotti, P.
Giannuzzi, G. Gibelli, A. Giordano, E. Giovannini, S. Gramenzi, F.
Ingrillì, S. Lombroso, F. Longaro, L. Magliani, C. Manes, R. Mangia,
G. Misuraca, R. Mocchegiani, A.P. Morciano, G. Occhi, M. Olivieri,
M. Palvarini, R. Panciarola, L. Pasetti, S. Pede, R. Pedretti, G.P.
Perini, F. Perticone, G. Pettinati, F. Plastina, M. Porcu, C. Porcellati,
F. Pozzar, G. Pulignano, C. Rapezzi, F. Rusconi, A. Sanna, M.
Santini, V. Santoro, S. Scalvini, F. Scapellato, A.M. Schillaci, C.
Schweiger, G. Sinagra, D. Staniscia, P. Tanzi, L. Tavazzi, E.
Uslenghi, G. Ventura, A. Vetrano, E. Zanelli; the Netherlands — M.
Baselier, P. Bruels, P.W.F. Bruggink, F. Den Hartog, P. Dunselman,
P. Fels, G. Geurts, H. Groeneveld, B. Hamer, N. Holwerda, J.
Kragten, G. Laarman, J. Levert, A. Liem, P. Lindner, H.R. Michels,
G. Paulussen, J.L. Posma, H.J. Schaafsma, W.M. Siesverda, L.C.
Slegers, P. van der Burgh, E.C.M. van der Velden, A.J.J. van Es, L.
van Kempen, H. van Kesteren, R. van Rijswijk, P. van Rossum, D.J.
Heijden, J.C.L. Wesdorp, A. Willems, A. Withagen; Norway — M.
Bjurstroem, A. Hervold, T.O. Klemsdal, K. Knutsen, T. Lappegaard, S.
Solheim, S. Toft, K.V. Tuseth, A. Westheim, T. Wessel-Aas; South
Africa — L.J. Burgess, P.J. Commerford, Y. Govender, E. Klug, P.
Manga, F. Maritz, D.P. Naidoo, L. Opie, H.W. Prozesky, D. Smith;
Spain — A. Aguilar Llopis, E. Asin Cardiel, V. Barrios Alonso, A.
Bayes De Luna, J. Bayon Fernandez, J.L. Diago Torrent, E. Galve
Basilio, M. Gil De La Peña, M. Gómez Gerboles, E. Homs Espinach, I.
Iglesias Garriz, J. Julia Gibergans, A. López Granados, A. Mallol
Kirchner, N. Manito Lorite, R. Masia Martorell, J. Orus, J. Padro
Dalmau, F. Pérez Villa, J. Roca Elias, E. Roig Minguell, J. Soler
Soler, F. Valles Belsue; Sweden — A. Andersson, H. Brodersson, S.
Ekdahl, J. Ellström, S. Hansen, J. Herlitz, L. Hjelmaeus, C.
Höglund, B. Karlsson, P. Katzman, L. Ljungdahl, M. Lundblad, H.
Tygesen, C. Wettervik, L. Winberg; United Kingdom — A. Baksi, A.
Blackwell, C. Cope, D. Connelly, T.R. Cripps, G. Dadahl, M.K. Ghosh,
S. Gibbs, S. Gupta, M.E. Heber, P.J.B. Hubner, G.D. Johnston, N.
Jones, J. Kooner, R. Levy, D. Lubel, P. Nicholls, A. Rozkovec, P.
Schofield, E. Smith, I.B. Squire, L.B. Tan, C. Welsh, S. Williams;
Executive Committee — J.N. Cohn (chair), G. Tognoni (co-chair), R.D.
Glazer, D. Spormann; Steering Committee — J.N. Cohn (chair), G.
Tognoni (co-chair), H. Krum, J. Vanhaecke, J. Widimsky, T. Haghfelt,
S. Majahalme, P. Lechat, K. Stumpe, L. Tan, C. Farsang, L. Tavazzi,
N.J. Holwerda, A. Westheim, L. Opie, A. Bayes de Luna, C. Höglund,
I. Anand; Study Coordination Centers — S. Ziesche, R. Latini, A.P.
Maggioni; End-Point Committee — P. Carson (chair), C. Opasich,
M. Scherillo, G. Sinagra, A. Volpe, C. O'Connor, I. Piña, F.
Tristani, L.W. Stevenson; Data Safety Monitoring Board — W. Parmley
(chair), M. Bobbio, D.J. van Veldhuisen, J. Abrams, D. DeMets; Echo
Core Laboratory — M. Wong, C. Höglund, L. Staszewsky, A. Volpi;
Neurohormone Laboratory — D. Judd, R. Latini, S. Masson.
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.