|
|
Irinotecan plus Cisplatin Compared with Etoposide
plus Cisplatin for Extensive Small-Cell Lung Cancer
Kazumasa Noda, M.D., Yutaka Nishiwaki, M.D., Masaaki
Kawahara, M.D., Shunichi Negoro, M.D., Takahiko Sugiura, M.D., Akira Yokoyama, M.D.,
Masahiro Fukuoka, M.D., Kiyoshi Mori, M.D., Koshiro Watanabe, M.D., Tomohide
Tamura, M.D., Seiichiro Yamamoto, Ph.D., Nagahiro Saijo, M.D., for the Japan
Clinical Oncology Group
ABSTRACT
Background Irinotecan
hydrochloride, a topoisomerase I inhibitor, is effective against
small-cell lung cancer. In a phase 2 study of irinotecan plus
cisplatin in patients with extensive small-cell lung cancer, there
was a high response rate and a promising median survival time.
Methods We conducted
a multicenter, randomized, phase 3 study in which we compared
irinotecan plus cisplatin with etoposide plus cisplatin in patients
with extensive (metastatic) small-cell lung cancer.
Results The planned
size of the study population was 230 patients, but enrollment was
terminated early because an interim analysis found a statistically
significant difference in survival between the patients assigned to
receive irinotecan and cisplatin and those assigned to receive
etoposide and cisplatin; as a result, only 154 patients were
enrolled. The median survival was 12.8 months in the
irinotecan-plus-cisplatin group and 9.4 months in the
etoposide-plus-cisplatin group (P=0.002 by the unadjusted log-rank
test). At two years, the proportion of patients surviving was 19.5
percent in the irinotecan-plus-cisplatin group and 5.2 percent in
the etoposide-plus-cisplatin group. Severe or life-threatening
myelosuppression was more frequent in the etoposide-plus-cisplatin group
than in the irinotecan-plus-cisplatin group, and severe or
life-threatening diarrhea was more frequent in the irinotecan-plus-cisplatin
group than in the etoposide-plus-cisplatin group.
Conclusions Irinotecan
plus cisplatin is an effective treatment for metastatic small-cell
lung cancer.
The usual chemotherapy for extensive
small-cell lung cancer is etoposide plus cisplatin or this
combination in alternation with a regimen of cyclophosphamide,
doxorubicin, and vincristine.1,2,3,4
In preliminary studies, irinotecan hydrochloride, a topoisomerase I
inhibitor, was effective against small-cell lung cancer,5
and a phase 2 study of irinotecan plus cisplatin yielded a rate of
complete response of 29 percent and an overall response rate of 86
percent (median survival, 13.2 months) in patients with extensive
small-cell lung cancer.6 For
these reasons, we conducted a randomized, phase 3 study to compare
irinotecan plus cisplatin with etoposide plus cisplatin in patients
with extensive small-cell lung cancer.
Methods
Patients
To be included in the study, patients had to have cytologically
or histologically confirmed small-cell lung cancer; extensive disease
(defined by distant metastasis, contralateral hilar-node metastasis,
or both; those with pleural effusion alone were excluded); no prior
radiotherapy, chemotherapy, or surgery; measurable lesions; an
Eastern Cooperative Oncology Group (ECOG) performance status of 0,
1, or 2; a life expectancy of at least three months; an age of 70
years or less; and adequate organ function. Staging of the tumor was
based on the results of physical examination, chest radiography,
fiberoptic bronchoscopy with biopsy and cytologic examination,
computed tomography (CT) of the chest and the brain, ultrasonography
or CT of the abdomen, radionuclide bone scanning, bone marrow
aspiration or biopsy, and other tests as needed. Adequate organ
function (adequate function of the bone marrow, liver, and kidney)
was defined as indicated by a leukocyte count of at least 4000 per
cubic millimeter, a platelet count of at least 100,000 per cubic
millimeter, a hemoglobin level of at least 9.5 g per deciliter (5.9
mmol per liter), aspartate aminotransferase and alanine
aminotransferase levels no higher than 100 IU per milliliter, a
serum creatinine level no higher than 1.2 mg per deciliter (106 Ámol
per liter), and a creatinine clearance of at least 60 ml per minute.
The exclusion criteria were infection, diarrhea, ileus,
interstitial pneumonitis, pulmonary fibrosis, uncontrolled diabetes
mellitus, myocardial infarction within the preceding three months,
massive pleural or peritoneal effusion, symptomatic brain metastases
requiring whole-brain irradiation or administration of corticosteroids,
a paraneoplastic syndrome, an active synchronous cancer, a metachronous
cancer within three disease-free years, and pregnancy or breast-feeding.
Treatment Assignment and Drug
Administration
The patients were randomly assigned to receive either a
combination of irinotecan and cisplatin or a combination of etoposide
and cisplatin by the minimization method of balancing the groups
according to the institution and the patients' performance status. Randomization
was performed at the Japan Clinical Oncology Group (JCOG) data
center according to the order in which information on enrollments
was received by telephone or fax. The regimen of irinotecan and
cisplatin consisted of four four-week cycles of 60 mg of irinotecan
per square meter of body-surface area on days 1, 8, and 15 and 60 mg
of cisplatin per square meter on day 1. The regimen of etoposide and
cisplatin consisted of four three-week cycles of 100 mg of etoposide
per square meter on days 1, 2, and 3 and 80 mg of cisplatin per
square meter on day 1. Both regimens required hydration and
administration of antiemetic drugs. If the leukocyte count fell
below 2000 per cubic millimeter or the neutrophil count fell below
1000 per cubic millimeter, recombinant human granulocyte
colony-stimulating factor was administered until the leukocyte or
neutrophil count was restored. Because not all patients received the
planned dose intensity (due to toxicity), we considered the planned
intensity of cisplatin to be 15 mg and 26.7 mg per square meter per
week in the irinotecan-plus-cisplatin group and the etoposide-plus-cisplatin
group, respectively.
Dose Modifications and Modifications in
the Treatment Schedule
Toxic effects were graded according to the JCOG Toxicity Criteria,7
in which a grade of 1 indicates a mild effect, grade 2 a moderate effect,
grade 3 a severe effect, and grade 4 a life-threatening effect.
Administration of irinotecan was skipped on day 8 or 15 if the
leukocyte count was 2000 per cubic millimeter or less, if the
platelet count was 50,000 per cubic millimeter or less, or if there
was diarrhea. Administration of subsequent cycles of irinotecan was
allowed when the leukocyte count reached at least 3500 per cubic
millimeter, the platelet count reached at least 100,000 per cubic
millimeter, and the diarrhea had subsided. The dose of irinotecan in
subsequent cycles was reduced by 10 mg per square meter from the
planned dose if there were grade 4 hematologic toxic effects or if
grade 2 or 3 diarrhea developed. Treatment was discontinued in
patients with grade 4 diarrhea.
Etoposide was discontinued if the leukocyte count was 3500 per
cubic millimeter or less, if the platelet count was 75,000 per cubic
millimeter or less, or if the serum creatinine level was 1.5 mg per
deciliter (132.6 Ámol per liter) or higher. In patients with grade 4
hematologic toxic effects, the doses of etoposide and cisplatin in
subsequent cycles were reduced to 75 percent of the planned doses.
In both study groups, the dose of cisplatin was reduced to 75
percent of the planned dose in patients with grade 2 renal toxic
effects. Subsequent cycles of treatment were suspended entirely in
patients with grade 2 hepatic toxic effects until the results of
liver-function tests were normal. Treatment was terminated in
patients with renal toxic effects rated grade 3 or higher, pulmonary
toxic effects rated grade 2 or higher, or hepatic toxic effects
rated grade 3 or higher.
A second randomization to evaluate subsequent thoracic
radiotherapy as a means of inhibiting local relapse was canceled
because of an inadequate number of eligible patients.
Evaluations
All the patients underwent weekly evaluations that included an
assessment of symptoms, a physical examination, chest radiography, a
complete blood count, blood-chemistry studies (including measurements of
aspartate aminotransferase and alanine aminotransferase, lactate
dehydrogenase, bilirubin, serum creatinine, blood urea nitrogen,
total protein, serum albumin, serum electrolytes, and calcium), and
urinalysis. Tumor response was evaluated according to World Health
Organization criteria8 and
was assessed by chest radiography or chest CT and by the same tests
used initially to stage the tumor. A complete response was defined
as the disappearance of all clinical and radiologic evidence of
tumor for at least four weeks; a partial response was defined as a
decrease of 50 percent or more in the sum of the products of the longest
perpendicular diameters of all measurable lesions for at least four
weeks; and progressive disease was defined as an increase of more
than 25 percent in the sum of the products of the perpendicular diameters
of all measurable lesions or the appearance of new lesions. All
other circumstances were considered to indicate no change. All the
observed responses were reviewed by an extramural panel at regular
study-group meetings. A planned quality-of-life study9 was
terminated because of poor compliance.
Study Design and Statistical Analysis
This trial was designed as a multicenter, prospective, randomized
phase 3 study. The study protocol was approved by the Clinical Trial
Review Committee of JCOG and the institutional review board of each
participating institution before the initiation of the study, and
all the patients provided written informed consent before
randomization in accordance with the policies of JCOG in effect in
1995, when enrollment began. The primary end point was overall
survival, and the secondary end points were the rates of complete
and overall response, progression-free survival, sites of relapse,
and toxicity. The sample size initially planned was 230 patients
from 54 participating sites, with 115 patients in each group. The
planned duration of accrual was 3 years, and the planned follow-up
time was 1.5 years. This sample size was designed to provide the
study with 80 percent power to detect an improvement of 9 months in
the median survival of the patients in the etoposide-plus-cisplatin
group and an improvement of 13 months in the median survival of
patients in the irinotecan-plus-cisplatin group (hazard ratio, 0.69)
with a one-sided type I error of 0.05.
All comparisons of patients' characteristics, prognostic
variables, response rates, and rates of toxic effects were performed
with Fisher's exact test, except for age, for which the t-test was
used. Survival was measured as the date of randomization to the
date of death or the date of the most recent follow-up. Progression-free
survival was measured as the date of randomization to the date of
the first observation of disease progression or the date of death
from any cause if there had been no progression. If there was no
progression and if the patient had not died, data on
progression-free survival were censored as of the date that the
absence of progression was confirmed. If a patient died without
information on progression, data on progression-free survival were
censored as of the last date on which progression could be ruled out
by review of follow-up forms. Survival curves were calculated by the
Kaplan–Meier method10
and compared with use of the log-rank test.
Two interim analyses were planned, with adjustment for multiple
comparisons taken into account by the method of Lan and DeMets.11
The O'Brien–Fleming type alpha spending function was used. The
first interim analysis was planned for the date on which half the
planned number of patients had been enrolled, and the second for the
date on which all the patients had been enrolled. The boundaries
were calculated with the use of computer programs provided by
Reboussin et al.12
The current study was designed and conducted on the basis of
one-sided testing, but the results are presented with two-sided P
values. Unadjusted P values are reported because of the conservative
spending function used.
All patient-information forms were collected and managed at the
data center. In-house interim monitoring was performed at the data center
to ensure the submission of data, the eligibility of the patients,
compliance with the protocol, safety, and progress of the study on
schedule. The monitoring reports were submitted to and reviewed by
an independent monitoring committee semiannually.
Results
Enrollment in the study began in November 1995. The first interim
analysis, performed in August 1998, suggested a difference in overall
survival between the two study groups; the monitoring committee
therefore recommended that the second interim analysis be performed
earlier than planned. The second analysis, performed in December
1998, found a significant difference in overall survival between the
two groups (P<0.001), and the monitoring committee therefore
recommended termination of the study. Enrollment was discontinued
and the study was terminated in January 1999.
Between November 1995 and November 1998, 154 patients were
enrolled in the study at 27 sites among the 54 institutions planned,
with 77 randomly assigned to receive irinotecan and cisplatin and
77 randomly assigned to receive etoposide and cisplatin (Table 1). All the
enrolled patients were included in the analyses of survival,
progression-free survival, and tumor response. However, two patients
in the irinotecan-plus-cisplatin group were given no chemotherapy,
one because of rapid progression of disease and the other because of
an acute gastric ulcer that was diagnosed immediately after
randomization. Both of these patients were excluded from the
analysis of toxicity. The average follow-up time was 16.8 months in
the irinotecan-plus-cisplatin group and 11.7 months in the
etoposide-plus-cisplatin group. None of the patients were lost to
follow-up.
|
Toxicity
Hematologic toxic effects are shown in Table 2. JCOG
grade 3 or 4 leukopenia and neutropenia and grade 3 or 4
thrombocytopenia were more frequent in the etoposide-plus-cisplatin
group than in the irinotecan-plus-cisplatin group. Grade 3 or 4
diarrhea occurred in 16.0 percent of the irinotecan-plus-cisplatin
group and in none of the etoposide-plus-cisplatin group
(P<0.001). Grade 1 or 2 diarrhea was also frequent in the
irinotecan-plus-cisplatin group. The incidence of nausea and
vomiting and other nonhematologic toxic effects did not differ
significantly between the two groups.
|
Major deviations from the protocol were the failure to reduce the
dose of chemotherapy despite the presence of grade 4 neutropenia (in
six patients in the irinotecan-plus-cisplatin group and seven in the
etoposide-plus-cisplatin group), administration of irinotecan
despite the presence of grade 1 or 2 diarrhea (in nine patients in
the irinotecan-plus-cisplatin group), continuation of the study
treatment despite grade 2 or 3 pulmonary toxic effects (in three
patients in the irinotecan-plus-cisplatin group and six in the
etoposide-plus-cisplatin group), and continuation of the study
treatment despite grade 3 hepatic toxic effects (in one patient in
the irinotecan-plus-cisplatin group and three in the
etoposide-plus-cisplatin group).
There were four treatment-related deaths, three in the
irinotecan-plus-cisplatin group and one in the
etoposide-plus-cisplatin group. In the irinotecan-plus-cisplatin
group, a 63-year-old man died of bleeding from a metastatic site in
the lung, a 62-year-old man died of sepsis associated with
neutropenia and diarrhea, and a 64-year-old woman died of pneumonia
associated with neutropenia. In the etoposide-plus-cisplatin group,
a 69-year-old man died of radiation pneumonitis after completion of
subsequent thoracic radiotherapy.
Delivery of Treatment
There were no significant differences between the two groups in
the delivery of treatment (Table 3). The
proportion of patients who received the planned four cycles of
chemotherapy was approximately 70 percent in each group. More
patients in the etoposide-plus-cisplatin group (38 percent) than in
the irinotecan-plus-cisplatin group (29 percent) completed their
assigned study treatment with no modifications in the doses or
delivery schedule. The dose intensity (the actual dose delivered as
a proportion of the planned dose) was 80.4 percent for irinotecan
and 95.3 percent for cisplatin in the group assigned to receive
irinotecan plus cisplatin and was 83.9 percent for etoposide and
84.6 percent for cisplatin in the group assigned to receive
etoposide plus cisplatin. The actual intensity of the dose of
cisplatin in the etoposide-plus-cisplatin group was 1.58 times that
in the irinotecan-plus-cisplatin group (22.6 vs. 14.3 mg per square
meter per week, respectively).
|
Rates of Response
The rate of complete response and the overall response rate were
2.6 percent (95 percent confidence interval, 0.3 to 9.1 percent) and
84.4 percent (95 percent confidence interval, 74.4 to 91.7 percent),
respectively, in the irinotecan-plus-cisplatin group and 9.1 percent
(95 percent confidence interval, 3.7 to 17.8 percent) and 67.5
percent (95 percent confidence interval, 55.9 to 77.8 percent),
respectively, in the etoposide-plus-cisplatin group (Table 4). The
rate of overall response in the irinotecan-plus-cisplatin group was
significantly higher than that in the etoposide-plus-cisplatin group
(P=0.02).
|
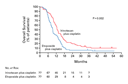
Overall Survival
As of March 2001, when the final analysis was conducted, the median
overall survival was 12.8 months (95 percent confidence interval,
11.7 to 15.2) in the irinotecan-plus-cisplatin group and 9.4 months
(95 percent confidence interval, 8.1 to 10.8) in the etoposide-plus-cisplatin
group; 70 patients in the irinotecan-plus-cisplatin group and 74 in
the etoposide-plus-cisplatin group died (P=0.002 by the log-rank
test) (Figure 1).
The rate of overall survival in the irinotecan-plus-cisplatin group
was 58.4 percent (95 percent confidence interval, 47.4 to 69.4
percent) at one year and 19.5 percent (95 percent confidence
interval, 10.6 to 28.3 percent) at two years; in the
etoposide-plus-cisplatin group, the rates of overall survival at
these time points were 37.7 percent (95 percent confidence interval,
26.8 to 48.5 percent) and 5.2 percent (95 percent confidence
interval, 0.2 to 10.2 percent). The risk of death in the
irinotecan-plus-cisplatin group relative to that in the
etoposide-plus-cisplatin group was 0.60 (95 percent confidence
interval, 0.43 to 0.83). Similar results were obtained in analyses
that adjusted for age, sex, performance status, and weight loss and
in an analysis that excluded the 23 patients randomly assigned to
the radiotherapy portion of the study, which was canceled.
|
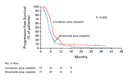
Progression-free Survival
The median known progression-free survival was 6.9 months (95
percent confidence interval, 6.1 to 7.3) in the irinotecan-plus-cisplatin
group and 4.8 months (95 percent confidence interval, 4.3 to 5.5)
in the etoposide-plus-cisplatin group. The rate of known progression-free
survival in the irinotecan-plus-cisplatin group was 65.3 percent (95
percent confidence interval, 54.3 to 76.3 percent) at six months and
12.5 percent (95 percent confidence interval, 4.9 to 20.1 percent)
at one year; in the etoposide-plus-cisplatin group the rates of
progression-free survival at these time points were 35.6 percent (95
percent confidence interval, 24.8 to 46.3 percent) and 7.9 percent
(95 percent confidence interval, 1.8 to 14.0 percent), respectively
(P=0.003 by the log-rank test) (Figure 2).
Progression was known to have occurred in 68 patients in the
irinotecan-plus-cisplatin group and 75 patients in the etoposide-plus-cisplatin
group. The relative risk of disease progression in the
irinotecan-plus-cisplatin group as compared with that in the
etoposide-plus-cisplatin group was 0.61 (95 percent confidence
interval, 0.44 to 0.84). The estimates of progression-free survival,
however, may be biased because information on progression was not
monitored continuously and because there were 10 instances of early
censoring because of death without data on progression (8 instances
in the irinotecan-plus-cisplatin group and 2 in the
etoposide-plus-cisplatin group).
|
Discussion
The current standard chemotherapy for extensive small-cell lung
cancer — a regimen of etoposide and cisplatin or this combination
alternating with a combination of cyclophosphamide, doxorubicin, and
vincristine — yields a median survival of 8 to 10 months and a
2-year survival rate of 10 percent. In this phase 3 study, 77
patients with metastatic small-cell lung cancer who were treated
with irinotecan plus cisplatin had a median survival of 12.8 months,
whereas the group that received etoposide plus cisplatin had a
median survival of 9.4 months (P=0.002). The overall rates of
survival in these two groups at two years were 19.5 percent and 5.2
percent, respectively.
Myelosuppression was the most frequent toxic effect in both groups
and was more frequent in the etoposide-plus-cisplatin group than in
the irinotecan-plus-cisplatin group. There was, however, a
significantly higher incidence of grade 3 or 4 diarrhea among the
patients who received irinotecan than among those who received
etoposide.
The three treatment-related deaths in the
irinotecan-plus-cisplatin group occurred during the first or second
cycle of treatment and were attributed to hematologic toxic effects
of the first cycle. Severe hematologic toxic effects, as well as
diarrhea, during the initial cycles of chemotherapy should therefore
be managed carefully. All cases of grade 1 to 4 diarrhea occurred
during the first and second cycles of irinotecan-plus-cisplatin treatment,
but early suspension of treatment prevented death associated with
diarrhea in all but one case, which involved a protocol violation in
which the patient was given irinotecan on day 8 of the first cycle
despite the presence of grade 1 diarrhea. We administered loperamide
hydrochloride or a Chinese herbal drug such as hange-shashin-to to
ameliorate the diarrhea at the discretion of the attending
physicians.
The proportion of patients who received all four cycles of
chemotherapy was similar in the two groups (approximately 70
percent), and thus the observed difference in survival is not
thought to be attributable to a difference in the actual delivery of
treatment.
Our study had several weaknesses. The planned second randomization
to allow us to assess the benefit of subsequent thoracic radiotherapy
was not completed; the planned quality-of-life study was not completed;
and full information concerning treatment after disease progression
was not available. The estimates of overall survival, however, should
be highly reliable because, as of March 2001 (the final analysis),
no patient had been lost to follow-up.
We consider that the trend toward a higher complete-response rate
in the etoposide-plus-cisplatin group than in the irinotecan-plus-cisplatin
group is due to chance. Although it is possible that these results occurred
by chance, we believe that the decision to terminate the trial early
was based on generally accepted scientific and ethical principles
and that, despite the small sample size, we can conclude that the
combination of irinotecan and cisplatin is an attractive option for
patients with metastatic small-cell lung cancer who have a good
performance status.
Supported in
part by grants-in-aid for cancer research and for the Second-Term
Comprehensive 10-Year Strategy for Cancer Control from the Ministry
of Health, Labor, and Welfare (Tokyo).
We are indebted to Ms. M. Imai and Ms. M. Niimi for data
management; to Dr. K. Yoshimura for analyses during periodic interim
monitoring; and to Dr. H. Fukuda for direction of the JCOG data
center and oversight of management of the study.
* Other participating institutions and
investigators are listed in the Appendix.
Source Information
From Kanagawa Cancer Center, Yokohama (K.N.); National Cancer
Center Hospital East, Chiba (Y.N.); National Kinki Central Hospital for Chest
Diseases, Osaka (M.K.); Osaka City General Hospital, Osaka (S.N.); Aichi Cancer
Center, Nagoya (T.S.); Niigata Cancer Center Hospital, Niigata (A.Y.); Kinki
University School of Medicine, Osaka (M.F.); Tochigi Cancer Center, Tochigi
(K.M.); Yokohama Municipal Citizen's Hospital, Yokohama (K.W.); National Cancer
Center Central Hospital, Tokyo (T.T., N.S.); and the Cancer Information and
Epidemiology Division, National Cancer Center Research Institute, Tokyo (S.Y.)
— all in Japan.
Address reprint requests to Dr. Saijo at the National Cancer
Center, Tsukiji 5-1-1, Chuo-ku, Tokyo 104-0045, Japan, or at [log in to unmask].
References
- Fukuoka
M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide,
doxorubicin, and vincristine versus cisplatin and etoposide versus
alternation of these regimens in small-cell lung cancer. J Natl Cancer
Inst 1991;83:855-861.
[Abstract]
- Roth BJ,
Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide,
doxorubicin, and vincristine versus etoposide and cisplatin versus
alternation of these two regimens in extensive small-cell lung cancer: a
phase III trial of the Southeastern Cancer Study Group. J Clin Oncol
1992;10:282-291.
[Abstract]
- Ihde DC.
Chemotherapy of lung cancer. N Engl J Med 1992;327:1434-1441.
[Medline]
- Aisner
J. Extensive-disease small-cell lung cancer: the thrill of victory; the
agony of defeat. J Clin Oncol 1996;14:658-665.
[Medline]
- Masuda N,
Fukuoka M, Kusunoki Y, et al. CPT-11: a new derivative of camptothecin for
the treatment of refractory or relapsed small-cell lung cancer. J Clin
Oncol 1992;10:1225-1229.
[Abstract]
- Kudoh S,
Fujiwara Y, Takada Y, et al. Phase II study of irinotecan combined with
cisplatin in patients with previously untreated small-cell lung cancer. J
Clin Oncol 1998;16:1068-1074.
[Abstract]
- Tobinai
K, Kohno A, Shimada Y, et al. Toxicity grading criteria of the Japan
Clinical Oncology Group. Jpn J Clin Oncol 1993;23:250-257.
[Medline]
- WHO
handbook for reporting the results of cancer treatment. No. 48. Geneva:
World Health Organization, 1979.
- Aaronson
NK, Ahmedzai S, Bergman B, et al. The European Organization for Research
and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in
international clinical trials in oncology. J Natl Cancer Inst
1993;85:365-376.
[Abstract]
- Kaplan
EL, Meier P. Nonparametric estimation from incomplete observations. J Am
Stat Assoc 1958;53:457-81.
- DeMets
DL, Lan KKG. Interim analysis: the alpha spending function approach. Stat
Med 1994;13:1341-1352.
[Medline]
- Reboussin
DM, DeMets DL, Kim KM, Lan KKG. Programs for computing group sequential
boundaries using the Lan-DeMets methods, version 2, 1998. (Accessed
December 7, 2001, at http://www.biostat.wisc.edu/landemets/.)
Appendix
This study was coordinated by the Japan Clinical Oncology Group
(M. Shimoyama, former chairperson) and was performed with the cooperation
of the following institutions and investigators: National Dohoku
Hospital, Hokkaido (T. Fujikane, K. Takahashi, and Y. Yamazaki);
Hokkaido Keiaikai Minami-ichijo Hospital, Hokkaido (A. Fujita);
Asahikawa Medical College Hospital, Hokkaido (Y. Osaki and Y.
Nishizaki); Yamagata Prefectural Central Hospital, Yamagata (T.
Tsukamoto); Tsukuba University Hospital, Ibaragi (S. Hasegawa and M.
Tajima); Tochigi Cancer Center, Tochigi (T. Hirose, S. Machida, and
M. Noda); National Nishi-Gunma Hospital, Gunma (S. Tsuchiya and H. Nakano);
Saitama Cancer Center, Saitama (S. Yoneda, H. Sakai, T. Ikeda, and
K. Kobayashi); National Cancer Center Hospital East, Chiba (F.
Houjo, R. Kakinuma, Y. Ohe, T. Matsumoto, H. Ohmatsu, K. Kodama, E.
Moriyama, and Y. Hosomi); National Cancer Center Central Hospital,
Tokyo (T. Shinkai, H. Kunitoh, K. Kubota, and I. Sekine);
International Medical Center of Japan, Tokyo (K. Kudo and Y.
Takeda); Kanagawa Cancer Center, Yokohama (I. Nomura, K. Yamada, F.
Oshita, Y. Kato, and M. Kondo); Yokohama Municipal Citizen's
Hospital, Yokohama (H. Kunikane and A. Nagatomo); Niigata Cancer
Center Hospital, Niigata (H. Tsukada, S. Mitsuma, and Y. Ichikawa);
Aichi Cancer Center, Nagoya (K. Yoshida and T. Hida); National Nagoya
Hospital, Nagoya (K. Nishiwaki and M. Hiraiwa); National Kinki
Central Hospital for Chest Diseases, Osaka (M. Ogawara, T.
Tsuchiyama, N. Kodama, K. Moriya, K. Okishio, N. Naka, S. Nobuyama,
and S. Yamamoto); Kinki University School of Medicine, Osaka (N.
Yamamoto, K. Nakagawa, T. Nogami, Y. Ieda, and M. Yoshida); Osaka
Prefectural Habikino Hospital, Osaka (I. Kawase, N. Masuda, T.
Nitta, and M. Kobayashi); Osaka City General Hospital, Osaka (K.
Takeda, N. Yoshimura, H. Uejima, N. Nishikubo, T. Nitta, N. Takifuji,
R. Miyaguchi, and K. Sugioka); National Toneyama Hospital for Chest
Diseases, Osaka (H. Nishikawa and K. Shinkawa); Hyogo Medical Center
for Adults, Hyogo (Y. Takada and T. Kadoh); Hyogo Medical College,
Hyogo (K. Higashino and A. Tonomura); Wakayama Rohsai Hospital,
Wakayama (T. Hoso and H. Minakada); Sasebo Municipal General
Hospital, Nagasaki (J. Araki, K. Yamaguchi, and K. Ohba); Kumamoto
Chuo Hospital, Kumamoto (T. Kiyama and Y. Yoshioka); and Kumamoto
Regional General Hospital, Kumamoto (H. Senba and T. Seto).
This article has been cited by other
articles:
- Carney,
D. N. (2002). Lung Cancer -- Time to Move on from Chemotherapy. N
Engl J Med 346: 126-128
[Full Text]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.