|
|
Plasma Homocysteine as a Risk Factor for Dementia
and Alzheimer's Disease
Sudha Seshadri, M.D., Alexa Beiser, Ph.D., Jacob Selhub,
Ph.D., Paul F. Jacques, Sc.D., Irwin H. Rosenberg, M.D., Ralph B. D'Agostino,
Ph.D., Peter W.F. Wilson, M.D., and Philip A. Wolf, M.D.
ABSTRACT
Background In
cross-sectional studies, elevated plasma homocysteine levels have
been associated with poor cognition and dementia. Studies of newly
diagnosed dementia are required in order to establish whether the
elevated homocysteine levels precede the onset of dementia or result
from dementia-related nutritional and vitamin deficiencies.
Methods A total of 1092
subjects without dementia (667 women and 425 men; mean age, 76
years) from the Framingham Study constituted our study sample. We
examined the relation of the plasma total homocysteine level
measured at base line and that measured eight years earlier to the
risk of newly diagnosed dementia on follow-up. We used multivariable
proportional-hazards regression to adjust for age, sex,
apolipoprotein E genotype, vascular risk factors other than
homocysteine, and plasma levels of folate and vitamins B12
and B6.
Results Over a
median follow-up period of eight years, dementia developed in 111
subjects, including 83 given a diagnosis of Alzheimer's disease. The
multivariable-adjusted relative risk of dementia was 1.4 (95 percent
confidence interval, 1.1 to 1.9) for each increase of 1 SD in the
log-transformed homocysteine value either at base line or eight
years earlier. The relative risk of Alzheimer's disease was 1.8 (95
percent confidence interval, 1.3 to 2.5) per increase of 1 SD at
base line and 1.6 (95 percent confidence interval, 1.2 to 2.1) per
increase of 1 SD eight years before base line. With a plasma
homocysteine level greater than 14 Ámol per liter, the risk of
Alzheimer's disease nearly doubled.
Conclusions An increased
plasma homocysteine level is a strong, independent risk factor for
the development of dementia and Alzheimer's disease.
Alzheimer's disease accounts for more than
70 percent of all cases of dementia, so it is important to identify
modifiable risk factors for the disease.1
During the past decade, there has been growing interest in vascular
factors that may underlie Alzheimer's disease. It is now recognized
that subjects with cardiovascular risk factors and a history of
stroke have an increased risk of both vascular dementia and
Alzheimer's disease.2,3,4
Plasma total homocysteine has recently emerged as a major vascular risk
factor. Elevated total homocysteine levels have been associated with
an increased risk of atherosclerotic sequelae, including death from
cardiovascular causes,5,6
coronary heart disease,6,7
carotid atherosclerosis,8 and
clinical stroke.9,10
These observations led to the hypothesis that elevated plasma
homocysteine may be a risk factor for dementia and Alzheimer's
disease. If this hypothesis is valid, it points to a modifiable risk
factor, since plasma homocysteine levels can be lowered by
supplementation with folic acid.11
Previous studies have reported an inverse association between
plasma total homocysteine levels and simultaneously assessed cognitive
function.12,13,14,15,16
Two case–control studies have found higher plasma homocysteine
levels in persons with Alzheimer's disease.17,18
However, in a prospective study plasma homocysteine levels were not
related to cognitive decline during follow-up in a community-based
sample.19
Elevated plasma homocysteine levels in subjects with cognitive
impairment or dementia might be the result of poor nutrition and
vitamin deficiencies.20
A prospective study should be able to show whether elevated plasma
homocysteine in cognitively intact adults is associated with an
increased risk of dementia and Alzheimer's disease on follow-up. We
therefore examined plasma total homocysteine in relation to newly
diagnosed dementia and Alzheimer's disease in the elderly,
population-based cohort of Framingham Study participants.
Methods
Subjects
The Framingham Study cohort has been evaluated biennially since
1948. Between 1976 and 1978, a total of 2611 subjects were enrolled in
a dementia-free cohort.21,22 At
the 20th biennial examination (between 1986 and 1990), 1592 subjects
from this cohort were alive and free of dementia and had follow-up
data for at least one year. Of these subjects, 1229 (77 percent)
underwent the 20th examination, and in 1092 participants (89 percent
of those examined), plasma total homocysteine levels were measured.
These 1092 subjects constituted our study sample. There were 667
women and 425 men, and their mean (▒SD) age was 76▒6 years
(range, 68 to 97). Informed consent was obtained from all study
subjects with the use of a consent form approved by the
institutional review board for human research at the Boston University
School of Medicine.
Diagnosis of New Cases of Dementia and
Alzheimer's Disease
Subjects in the cohort that was free of dementia at inception
have been monitored with published surveillance techniques since 1978
for the development of stroke or dementia.21,22
Methods have included a screening Folstein Mini–Mental State
Examination23
at each biennial evaluation, followed by annual neurologic and neuropsychological
assessment of subjects with suspected cognitive impairment.
The final diagnosis of dementia was made by a committee,
comprising at least two neurologists and a neuropsychologist, that
determined the type of dementia and the date of diagnosis. All
available information was used to evaluate participants with
suspected dementia, including serial neurologic and
neuropsychological assessments, a telephone interview with a family
member or care giver, medical records, imaging studies, and autopsy
data when available. The review committee was unaware of the
subjects' plasma homocysteine levels. The diagnosis of dementia was
made according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders,
fourth edition24;
our definition also required a duration of symptoms greater than six
months, and a score for severity of dementia of 1 or higher on the
Clinical Dementia Rating scale.25
Alzheimer's disease was diagnosed when subjects met the criteria of
the National Institute of Neurological and Communicative Disorders
and Stroke and the Alzheimer's Disease and Related Disorders
Association for definite, probable, or possible Alzheimer's disease.26
Plasma Homocysteine
Plasma total homocysteine levels were measured in all subjects
at the 20th biennial examination (base line). An earlier measure from
the 16th biennial examination (performed between 1979 and 1982,
approximately eight years before base line) was also available for
935 of the subjects (86 percent). All plasma specimens were stored
at or below –20░C. Homocysteine levels were determined with the use
of high-performance liquid chromatography with fluorometric
detection.27
The coefficient of variation for this assay was 9 percent.28
Apolipoprotein E Genotypes
Data on the apolipoprotein E (APOE)
genotype were available for 1012 of the subjects (93 percent). The
presence of particular alleles was determined by means of
isoelectric focusing of the plasma and confirmed by DNA genotyping.29,30
Participants were divided into two groups, one comprising persons
with an APOE ![]() 4 allele (
4 allele (![]() 2/
2/![]() 4,
4, ![]() 3/
3/![]() 4, or
4, or ![]() 4/
4/![]() 4 genotype) and another comprising those without an APOE
4 genotype) and another comprising those without an APOE ![]() 4
allele.
4
allele.
Vitamin Levels
Plasma concentrations of folate, cyanocobalamin (vitamin B12),
and pyridoxal-5'-phosphate (the coenzyme form of vitamin B6)
were estimated at the 20th biennial examination. Plasma folate was
measured by a microbial (Lactobacillus casei)
assay with a 96-well plate and manganese supplementation31;
plasma vitamin B12 levels were estimated with the use of
a radioassay kit (Magic, Ciba–Corning, Medfield, Mass.); and
pyridoxal-5'-phosphate was measured by the tyrosine decarboxylase
apoenzyme method.32
Coefficients of variation for these assays were 13 percent for plasma
folate, 7 percent for cyanocobalamin, and 16 percent for
pyridoxal-5'-phosphate.28
Because of insufficient plasma samples, the vitamin levels were not
determined for all patients. Of the subjects with measurements of
plasma homocysteine, 85 percent had measurements of vitamin B12,
92 percent had measurements of vitamin B6, and 98 percent
had measurements of folate.
Definition of Additional Risk Factors
Risk factors that could potentially confound the relation between
plasma homocysteine and dementia or Alzheimer's disease were defined
with the use of data collected at the 20th biennial examination.
When appropriate, data from earlier biennial examinations were also
used. Educational status was dichotomized at the level of
high-school completion. We adjusted the analyses for cigarette smoking
using two variables: current smoking status (smoker or nonsmoker)
and lifetime exposure to cigarette smoke (<5.0 pack-years, 5.0 to
29.9 pack-years, or ![]() 30.0 pack-years). Alcohol
intake was categorized in terms of the number of drinks per day:
zero, less than one, one to two, or more than two.33
Diabetes mellitus was defined by a recorded casual blood glucose
level of at least 200 mg per deciliter (11.1 mmol per liter), a
previous diagnosis of diabetes mellitus, or the use of a
hypoglycemic agent or insulin. Systolic blood pressure and body-mass
index (the weight in kilograms divided by the square of the height
in meters) were treated as continuous variables.
30.0 pack-years). Alcohol
intake was categorized in terms of the number of drinks per day:
zero, less than one, one to two, or more than two.33
Diabetes mellitus was defined by a recorded casual blood glucose
level of at least 200 mg per deciliter (11.1 mmol per liter), a
previous diagnosis of diabetes mellitus, or the use of a
hypoglycemic agent or insulin. Systolic blood pressure and body-mass
index (the weight in kilograms divided by the square of the height
in meters) were treated as continuous variables.
Statistical Analysis
The distribution of plasma homocysteine levels in the population
was positively skewed. The use of natural-log–transformed values
provided the best-fitting model for analyses in which the plasma
homocysteine level was treated as a continuous variable. Plasma
homocysteine levels were also evaluated with a quartile-based analysis.
Since homocysteine levels increase markedly with age,28,34,35
the quartiles were defined in an age-specific manner for each of
several five-year age categories.
Cox proportional-hazards regression models36
were used to examine the relation between the homocysteine level and
the incidence of dementia and Alzheimer's disease during follow-up,
after adjustment for age (in one-year increments), sex, and APOE genotype (with or without
an APOE ![]() 4 allele).37 In
supplementary analyses, we also adjusted for vitamin levels and
other covariates. Subjects were followed for new cases of dementia
from the date of their 20th biennial examination until December 31,
2000. For the analysis of new cases of Alzheimer's disease, data for
subjects in whom other types of dementia developed were censored at
the date of the diagnosis of dementia, since the diagnostic
categories were mutually exclusive. Subjects who had a stroke during
the study period were not excluded, since such an event could be
part of the causal chain between an elevated plasma homocysteine level
and the development of dementia. All statistical analyses were
performed with the use of SAS software (SAS Institute, Cary, N.C.).
4 allele).37 In
supplementary analyses, we also adjusted for vitamin levels and
other covariates. Subjects were followed for new cases of dementia
from the date of their 20th biennial examination until December 31,
2000. For the analysis of new cases of Alzheimer's disease, data for
subjects in whom other types of dementia developed were censored at
the date of the diagnosis of dementia, since the diagnostic
categories were mutually exclusive. Subjects who had a stroke during
the study period were not excluded, since such an event could be
part of the causal chain between an elevated plasma homocysteine level
and the development of dementia. All statistical analyses were
performed with the use of SAS software (SAS Institute, Cary, N.C.).
Results
Base-Line Characteristics
The base-line characteristics of the subjects are presented in
Table 1
(further information may be found in Supplementary
Appendix 1, available with the full text of this article at
http://www.nejm.org). Mild-to-moderate elevation of the plasma homocysteine
level (>14 Ámol per liter) was present in 30 percent of the
subjects. None of the subjects had severe hyperhomocysteinemia
(plasma homocysteine, >100 Ámol per liter). The mean plasma
homocysteine level within each of the five-year age groups is shown
in Table 2.
The correlation between the base-line plasma homocysteine level in a
given subject and the level measured eight years earlier was
calculated for the 935 subjects (571 women and 364 men) for whom
both measurements were available (Pearson r=0.47, P<0.001).
|
|
|
Dementia, Alzheimer's Disease, and Plasma Homocysteine
Over a median follow-up period of 8 years (range, 1 to 13), dementia
developed in 111 subjects (10.2 percent; 74 women and 37 men), and
83 of these subjects (62 women and 21 men) were given a diagnosis of
Alzheimer's disease. In five subjects, the clinical diagnosis of
Alzheimer's disease was confirmed at autopsy (definite Alzheimer's
disease). The diagnosis was probable Alzheimer's disease for 67
subjects and possible Alzheimer's disease for 11 subjects. Other
types of dementia diagnosed in the study population included
vascular dementia in 11 subjects, non-Alzheimer's degenerative
dementias in 11 subjects, and other types of dementia in 6 subjects.
The absence of Alzheimer's disease was confirmed at autopsy in 14
subjects.
The overall results relating the plasma homocysteine level to
the development of any dementia and to the development of Alzheimer's
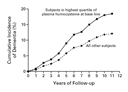
disease are shown in Table 3 and Table 4 and in Figure 1. After
adjustment for the age, sex, and APOE
genotype, the relative risks of dementia and Alzheimer's disease,
for each increase of 1 SD in log-transformed base-line homocysteine
value, were 1.3 (95 percent confidence interval, 1.1 to 1.6) and 1.4
(95 percent confidence interval, 1.2 to 1.7), respectively.
Hyperhomocysteinemia (plasma homocysteine, >14 Ámol per liter)8,18
was correspondingly associated with an increased risk of dementia
(relative risk, 1.9; 95 percent confidence interval, 1.3 to 2.8) and
Alzheimer's disease (relative risk, 1.9; 95 percent confidence
interval, 1.2 to 3.0). An increase in the plasma homocysteine level
of 5 Ámol per liter increased the multivariable-adjusted risk
of Alzheimer's disease by 40 percent (P<0.001). We did not find
evidence of modification of this effect by age or sex.
|
|
|
Effect of Vitamin Levels
Low serum levels of certain B vitamins (folate and vitamins B12
and B6) have been associated with elevated plasma homocysteine levels
in several studies and with an increased risk of dementia in a few
investigations.38,39,40,41,42 In
our study, the observed association between plasma homocysteine and
risk of dementia was not significantly altered by adjustment for the
plasma levels of these vitamins (Table 3).
Furthermore, after adjustment for age, sex, and APOE genotype, none of these vitamin
levels were independently related to the risk of dementia or
Alzheimer's disease (data not shown).
Additional Covariates
The observed association between the plasma homocysteine level
and dementia or Alzheimer's disease was not diminished by adjustment
for educational status, systolic blood pressure, smoking status, alcohol
intake, presence or absence of diabetes, body-mass index, or
presence or absence of a history of stroke (Table 3). Serum
creatinine was measured at the 15th biennial examination, and cholesterol
and thyrotropin were measured at the 20th biennial examination.
Adjustment for these additional variables did not alter our results
(data not shown).
Varying the Diagnostic Criteria for
Alzheimer's Disease
Higher plasma homocysteine levels have been related to an
increased risk of stroke.8,10 To
address the possibility that the association we observed between
plasma homocysteine and Alzheimer's disease resulted from the
inclusion of subjects who might have vascular dementia rather than
Alzheimer's disease, we evaluated separately the association between
base-line plasma homocysteine levels and a diagnosis of definite or
probable Alzheimer's disease after excluding subjects with a
diagnosis of possible Alzheimer's disease. The relative risk per
increment of 1 SD in the log-transformed base-line homocysteine
value remained essentially unchanged at 1.4 (95 percent confidence
interval, 1.2 to 1.7).
Association with Earlier Homocysteine
Levels
Unlike stroke or myocardial infarction, clinical dementia begins
insidiously. It may therefore be difficult to exclude subjects in
whom the disease is incipient at base line. However, subjects who
were free of clinical dementia at base line were most likely free of
even incipient disease eight years earlier, at the examination from
which we derived the previous plasma homocysteine measurement. We
examined the relation between the plasma homocysteine level eight
years before base line and the risk of newly diagnosed dementia or
Alzheimer's disease during the follow-up period between the
base-line examination and December 31, 2000. Again, we found a
strong association (Table
3), indicating that the elevation of the plasma homocysteine
level occurred well before the onset of clinical manifestations.
Quartile-Specific Analysis
Examination of the risks of dementia and Alzheimer's disease in
age-specific quartiles of plasma homocysteine levels suggested that
subjects with levels in the highest quartile (according to the
cutoff points in Table
2) had the highest risk of dementia and Alzheimer's disease.
When both measurements of plasma homocysteine were considered, this
subgroup had about twice the risk of all other subjects (Table 4 and Figure 1).
Although the effect of the homocysteine level was smaller in the
second and third quartiles, we did not find evidence of a specific
threshold. When the subjects whose base-line levels were in the
lowest age-specific quartile were used as the reference group, the
relative risk of Alzheimer's disease was 1.2 (95 percent confidence
interval, 0.6 to 2.2) for subjects in the second quartile, 1.3 (95
percent confidence interval, 0.6 to 2.5) for subjects in the third
quartile, and 2.2 (95 percent confidence interval, 1.2 to 4.1) for
subjects in the fourth quartile. Subjects whose plasma homocysteine
levels were consistently high (in the fourth quartile at both the
16th and 20th examinations) had the highest risk.
Population Attributable Risk
In our population, the risk of Alzheimer's disease attributable
to a plasma homocysteine level in the highest age-specific quartile was
estimated, with the use of standard techniques,43 at
16 percent. In the same population, 21 percent of subjects had at
least one APOE ![]() 4 allele, and the age- and sex-adjusted relative risk
of Alzheimer's disease associated with the presence of this allele
was 2.3 (95 percent confidence interval, 1.5 to 3.7); thus, there
was a 21 percent risk of Alzheimer's disease attributable to the
presence of an APOE
4 allele, and the age- and sex-adjusted relative risk
of Alzheimer's disease associated with the presence of this allele
was 2.3 (95 percent confidence interval, 1.5 to 3.7); thus, there
was a 21 percent risk of Alzheimer's disease attributable to the
presence of an APOE ![]() 4 genotype.
4 genotype.
Discussion
The results of our prospective, observational study indicate that
there is a strong, graded association between plasma total homocysteine
levels and the risk of dementia and Alzheimer's disease. An
increment in the plasma homocysteine level of 5 Ámol per liter
increased the risk of Alzheimer's disease by 40 percent. A plasma
homocysteine level in the highest age-specific quartile doubled the
risk of dementia or Alzheimer's disease. A similar result was found
when the single criterion of hyperhomocysteinemia (base-line plasma
homocysteine, >14 Ámol per liter) was used. The magnitude of this
effect is similar to the magnitude of the increases in the risks of
death from cardiovascular causes and stroke associated with a
similar increment in the plasma homocysteine level, which have been
previously described in the Framingham cohort.6,10
The observed association appeared to be independent of age, sex,
APOE genotype, plasma vitamin
levels, and other putative risk factors for dementia and Alzheimer's
disease. The prospective nature of this study and the strong
association between newly diagnosed dementia and Alzheimer's disease
and plasma homocysteine levels measured eight years before base line
suggest that the elevation in the homocysteine level preceded the onset
of dementia. Finally, subjects with a sustained elevation of plasma
homocysteine had the greatest risk of dementia.
Two case–control studies have specifically addressed the relation
between homocysteine levels and the risk of Alzheimer's disease.17,18
Both studies found a significant elevation of the serum homocysteine
level in patients with Alzheimer's disease as compared with
age-matched controls. A report from the Rotterdam Study did not show
an association between the base-line homocysteine level and a
decline in the score on the Mini–Mental State Examination, perhaps
because the follow-up period was only 2.7 years.19 In
our study population, an elevated homocysteine level at base line
was related to a decline in the scores on the Mini–Mental State
Examination, but only after a follow-up period of at least four
years (data not shown).
Elevated plasma homocysteine levels are associated with carotid
atherosclerosis and an increased risk of stroke.8,10
Atherosclerosis and stroke, in turn, increase the risk of clinical
Alzheimer's disease.2,4
Hyperhomocysteinemia has been related to cerebral microangiopathy,44
endothelial dysfunction,45
impaired nitric oxide activity,46
and increased oxidative stress47 —
all factors associated with the aging of the brain.48,49
Increased concentrations of homocysteic acid, an N-methyl-D-aspartate receptor
agonist and a metabolite of homocysteine, may result in excitotoxic
damage to neurons.50
Homocysteine promotes copper-mediated and ![]() -amyloid-peptide–mediated toxic effects in neuronal cell
cultures51
and induces apoptosis in hippocampal neurons in rats.52
-amyloid-peptide–mediated toxic effects in neuronal cell
cultures51
and induces apoptosis in hippocampal neurons in rats.52
The strengths of our investigation include its prospective design,
the large community-based sample, the long follow-up period, and
the availability of prestudy plasma homocysteine levels and
base-line values for plasma B vitamins and other covariates. A
limitation of this study is the lack of racial diversity in the
overwhelmingly white Framingham cohort. It is possible that our use
of samples obtained from nonfasting subjects resulted in estimates
of plasma homocysteine levels that were up to 20 percent higher than
they would have been in fasting subjects,53
but any increase in the variability in plasma homocysteine values caused
by this approach is likely to be random and is unlikely to have
altered the results.
Vitamin therapy with folic acid, alone or in combination with
vitamins B6 and B12, and dietary supplementation
with enriched cereal-grain products and breakfast cereals containing
folate can reduce plasma homocysteine levels.54,55,56
The U.S. government now mandates folic acid fortification of the
food supply.55
Current plasma homocysteine levels in the Framingham Study population
are significantly lower than those that were estimated at the 16th
and 20th biennial examinations.56
However, only 20 cases of dementia were diagnosed between 1997 and
the time the levels were remeasured, and therefore it is not
possible to assess the effect of recent increases in folic acid
fortification on the risk of dementia in this cohort. Furthermore,
since there have been no prospective trials of the effect of vitamin
supplementation on the incidence of dementia, our findings cannot be
used as a basis for setting health policy or treatment
recommendations.
The relation between elevated plasma homocysteine levels and dementia
must be evaluated in other cohort studies. If such studies confirm
our findings, proof of a causal association between plasma
homocysteine and the development of dementia and Alzheimer's disease
will require further elucidation of the pathophysiologic mechanisms
and direct evidence from controlled clinical trials in humans that
interventions that reduce plasma homocysteine levels can reduce the
risk of clinical dementia and Alzheimer's disease.
Supported by
the Framingham Heart Study's National Heart, Lung, and Blood
Institute contract (N01-HC-38038) and by grants (NIA-5R01-AG08122-11 and
NIA-5R01-AG16495-02) from the National Institute on Aging and a
grant (NINDS-5R01-NS17950-19) from the National Institute of
Neurological Disorders and Stroke.
Source Information
From the Department of Neurology (S.S., P.A.W.) and the Department
of Medicine (P.W.F.W.), Boston University School of Medicine; the Department of
Epidemiology and Biostatistics, Boston University School of Public Health
(A.B.); the Jean Mayer U.S. Department of Agriculture Human Nutrition Research
Center on Aging, Tufts University (J.S., P.F.J., I.H.R.); and the Department of
Mathematics and Statistics, Boston University (R.B.D.) — all in Boston.
Address reprint requests to Dr. Wolf at the Department of
Neurology (Neurological Epidemiology and Genetics), Boston University School of
Medicine, 715 Albany St., B-608, Boston, MA 02118-2526, or at [log in to unmask].
References
- Brookmeyer
R, Gray S, Kawas C. Projections of Alzheimer's disease in the United
States and the public health impact of delaying disease onset. Am J Public
Health 1998;88:1337-1342.[Abstract]
- Hofman
A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and
prevalence of dementia and Alzheimer's disease in the Rotterdam Study.
Lancet 1997;349:151-154.[Medline]
- Breteler
MM. Vascular risk factors for Alzheimer's disease: an epidemiologic
perspective. Neurobiol Aging 2000;21:153-160.[Medline]
- Snowdon
DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain
infarction and the clinical expression of Alzheimer disease: the Nun
Study. JAMA 1997;277:813-817.[Medline]
- Bots ML,
Launer LJ, Lindemans J, Hofman A, Grobbee DE. Homocysteine,
atherosclerosis and prevalent cardiovascular disease in the elderly: the
Rotterdam Study. J Intern Med 1997;242:339-347.[Medline]
- Bostom
AG, Silbershatz H, Rosenberg IH, et al. Nonfasting plasma total
homocysteine levels and all-cause and cardiovascular disease mortality in
elderly Framingham men and women. Arch Intern Med 1999;159:1077-1080.[Medline]
- Stampfer
MJ, Malinow MR, Willett WC, et al. A prospective study of plasma
homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA
1992;268:877-881.[Medline]
- Selhub
J, Jacques PF, Bostom AG, et al. Association between plasma homocysteine
concentrations and extracranial carotid-artery stenosis. N Engl J Med
1995;332:286-291.[Abstract/Full
Text]
- Perry
IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective
study of serum total homocysteine concentration and risk of stroke in
middle-aged British men. Lancet 1995;346:1395-1398.[Medline]
- Bostom
AG, Rosenberg IH, Silbershatz H, et al. Nonfasting plasma total
homocysteine levels and stroke incidence in elderly persons: the
Framingham Study. Ann Intern Med 1999;131:352-355.[Medline]
- Wald DS,
Bishop L, Wald NJ, et al. Randomized trial of folic acid supplementation
and serum homocysteine levels. Arch Intern Med 2001;161:695-700.[Medline]
- Lindenbaum
J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by
cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J
Med 1988;318:1720-1728.[Abstract]
- Bell IR,
Edman JS, Selhub J, et al. Plasma homocysteine in vascular disease and in
nonvascular dementia of depressed elderly people. Acta Psychiatr Scand
1992;86:386-390.[Medline]
- Riggs
KM, Spiro A III, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6,
folate, and homocysteine to cognitive performance in the Normative Aging
Study. Am J Clin Nutr 1996;63:306-314.[Abstract]
- Lehmann
M, Gottfries CG, Regland B. Identification of cognitive impairment in the
elderly: homocysteine is an early marker. Dement Geriatr Cogn Disord
1999;10:12-20.[Medline]
- Morris
MS, Jacques PF, Rosenberg IH, Selhub J. Hyperhomocysteinemia associated
with poor recall in the third National Health and Nutrition Examination
Survey. Am J Clin Nutr 2001;73:927-933.[Abstract/Full
Text]
- McCaddon
A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in
senile dementia of Alzheimer type. Int J Geriatr Psychiatry
1998;13:235-239.[Medline]
- Clarke
R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12,
and serum total homocysteine levels in confirmed Alzheimer's disease. Arch
Neurol 1998;55:1449-1455.[Medline]
- Kalmijn
S, Launer LJ, Lindemans J, Bots ML, Hofman A, Breteler MM. Total homocysteine
and cognitive decline in a community-based sample of elderly subjects: the
Rotterdam Study. Am J Epidemiol 1999;150:283-289.[Abstract]
- Diaz-Arrastia
R. Hyperhomocysteinemia: a new risk factor for Alzheimer disease? Arch
Neurol 1998;55:1407-1408.[Medline]
- Bachman
DL, Wolf PA, Linn RT, et al. Incidence of dementia and probable
Alzheimer's disease in a general population: the Framingham Study.
Neurology 1993;43:515-519.[Abstract]
- Seshadri
S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer's
disease: the impact of mortality on risk estimates in the Framingham
Study. Neurology 1997;49:1498-1504.[Abstract]
- Folstein
MF, Folstein SE, McHugh PR. "Mini-Mental State": a practical
method for grading the cognitive state of patients for the clinician. J
Psychiatr Res 1975;12:189-198.[Medline]
- Diagnostic
and statistical manual of mental disorders, 4th ed.: DSM-IV. Washington,
D.C.: American Psychiatric Association, 1994:143-6.
- Berg L.
Clinical Dementia Rating (CDR). Psychopharmacol Bull 1988;24:637-639.[Medline]
- McKhann
G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical
diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under
the auspices of Department of Health and Human Services Task Force on
Alzheimer's Disease. Neurology 1984;34:939-944.[Abstract]
- Araki A,
Sako Y. Determination of free and total homocysteine in human plasma by
high-performance liquid chromatography with fluorescence detection. J
Chromatogr 1987;422:43-52.[Medline]
- Selhub
J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake
as primary determinants of homocysteinemia in an elderly population. JAMA
1993;270:2693-2698.[Medline]
- Ordovas
JM, Litwack-Klein L, Wilson PW, Schaefer MM, Schaefer EJ. Apolipoprotein E
isoform phenotyping methodology and population frequency with
identification of apoE1 and apoE5 isoforms. J Lipid Res 1987;28:371-380.[Abstract]
- Welty
FK, Lahoz C, Tucker KL, Ordovas JM, Wilson PW, Schaefer EJ. Frequency of
ApoB and ApoE gene mutations as causes of hypobetalipoproteinemia in the
Framingham offspring population. Arterioscler Thromb Vasc Biol
1998;18:1745-1751.[Abstract/Full
Text]
- Horne
DW, Patterson D. Lactobacillus casei microbiological assay of folic acid
derivatives in 96-well microtiter plates. Clin Chem 1988;34:2357-2359.[Abstract]
- Shin YS,
Rasshofer R, Friedrich B, Endres W. Pyridoxal-5'-phosphate determination
by a sensitive micromethod in human blood, urine and tissues: its relation
to cystathioninuria in neuroblastoma and biliary atresia. Clin Chim Acta
1983;127:77-85.[Medline]
- Elias
PK, Elias MF, D'Agostino RB, Silbershatz H, Wolf PA. Alcohol consumption
and cognitive performance in the Framingham Heart Study. Am J Epidemiol
1999;150:580-589.[Abstract]
- Nilsson
K, Gustafson L, Faldt R, et al. Hyperhomocysteinaemia -- a common finding
in a psychogeriatric population. Eur J Clin Invest 1996;26:853-859.[Medline]
- Rasmussen
K, Moller J, Lyngbak M, Pedersen AM, Dybkjaer L. Age- and gender-specific
reference intervals for total homocysteine and methylmalonic acid in
plasma before and after vitamin supplementation. Clin Chem 1996;42:630-636.[Abstract]
- Cox DR,
Oakes D. Analysis of survival data. London: Chapman & Hall, 1984.
- Myers
RH, Schaefer EJ, Wilson PW, et al. Apolipoprotein E epsilon4 association
with dementia in a population-based study: the Framingham Study. Neurology
1996;46:673-677.[Abstract]
- Cole MG,
Prchal JF. Low serum vitamin B12 in Alzheimer-type dementia. Age Ageing
1984;13:101-105.[Abstract]
- Karnaze
DS, Carmel R. Low serum cobalamin levels in primary degenerative dementia:
do some patients harbor atypical cobalamin deficiency states? Arch Intern
Med 1987;147:429-431.[Medline]
- Ikeda T,
Furukawa Y, Mashimoto S, Takahashi K, Yamada M. Vitamin B12 levels in
serum and cerebrospinal fluid of people with Alzheimer's disease. Acta
Psychiatr Scand 1990;82:327-329.[Medline]
- Levitt
AJ, Karlinsky H. Folate, vitamin B12 and cognitive impairment in patients
with Alzheimer's disease. Acta Psychiatr Scand 1992;86:301-305.[Medline]
- Wang HX,
Wahlin A, Basun H, Fastbom J, Winblad B, Fratiglioni L. Vitamin B(12) and
folate in relation to the development of Alzheimer's disease. Neurology
2001;56:1188-1194.[Abstract/Full
Text]
- Levin
ML. The occurrence of lung cancer in man. Acta Unio Int Contr Cancrum
1953;9:531-41.
- Fassbender
K, Mielke O, Bertsch T, Nafe B, Froschen S, Hennerici M. Homocysteine in
cerebral macroangiography and microangiopathy. Lancet 1999;353:1586-1587.[Medline]
- Welch
GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med
1998;338:1042-1050.[Full
Text]
- Chao CL,
Kuo TL, Lee YT. Effects of methionine-induced hyperhomocysteinemia on
endothelium-dependent vasodilation and oxidative status in healthy adults.
Circulation 2000;101:485-490.[Abstract/Full
Text]
- Starkebaum
G, Harlan JM. Endothelial cell injury due to copper-catalyzed hydrogen
peroxide generation from homocysteine. J Clin Invest 1986;77:1370-1376.[Medline]
- McCann
SM. The nitric oxide hypothesis of brain aging. Exp Gerontol
1997;32:431-440.[Medline]
- Beal MF.
Aging, energy, and oxidative stress in neurodegenerative diseases. Ann
Neurol 1995;38:357-366.[Medline]
- Lipton
SA, Kim WK, Choi YB, et al. Neurotoxicity associated with dual actions of
homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S
A 1997;94:5923-5928.[Abstract/Full
Text]
- White
AR, Huang X, Jobling MF, et al. Homocysteine potentiates copper- and
amyloid beta peptide-mediated toxicity in primary neuronal cultures:
possible risk factors in the Alzheimer's-type neurodegenerative pathways.
J Neurochem 2001;76:1509-1520.[Abstract/Full
Text]
- Kruman
II, Culmsee C, Chan SL, et al. Homocysteine elicits a DNA damage response
in neurons that promotes apoptosis and hypersensitivity to excitotoxicity.
J Neurosci 2000;20:6920-6926.[Abstract/Full
Text]
- Perry
DJ. Hyperhomocysteinaemia. Baillieres Best Pract Res Clin Haematol
1999;12:451-477.[Medline]
- Homocysteine
Lowering Trialists' Collaboration. Lowering blood homocysteine with folic
acid based supplements: meta-analysis of randomised trials. BMJ
1998;316:894-898.[Abstract/Full
Text]
- Food
Standards: amendment of standards of identity for enriched grain products
to require additional folic acid. Fed Regist 1996;61:8781-8797.
- Jacques
PF, Selhub J, Bostom AG, Wilson PWF, Rosenberg IH. The effect of folic
acid fortification on plasma folate and total homocysteine concentrations.
N Engl J Med 1999;340:1449-1454.[Abstract/Full
Text]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.