|
|
Obesity
Susan Z. Yanovski, M.D., and Jack A. Yanovski, M.D., Ph.D.
Overweight and obesity are the most common nutritional disorders
in the United States, affecting the majority of adults in the country.
Given a normal body-mass index (defined as the weight in kilograms
divided by the square of the height in meters) ranging from 18.5 to
24.9, 34 percent of the adult population is overweight (body-mass
index, 25 to 29.9), and another 27 percent is obese (body-mass
index, ![]() 30).1 The
prevalence of obesity has increased by more than 75 percent since
1980.2
The prevalence of overweight in children and adolescents (defined as
a body-mass index in the 95th percentile or higher for age and sex)3 has
more than doubled since 1976.4
30).1 The
prevalence of obesity has increased by more than 75 percent since
1980.2
The prevalence of overweight in children and adolescents (defined as
a body-mass index in the 95th percentile or higher for age and sex)3 has
more than doubled since 1976.4
Health care professionals should be concerned about overweight
and obesity because of the well-established relations between excess
body weight and such medical conditions as type 2 diabetes, hypertension,
and osteoarthritis.5
Evidence-based guidelines issued by the National Institutes of
Health call for weight loss both in obese persons and in overweight
persons with two or more risk factors for obesity-related diseases.6
Medications for the treatment of obesity are currently approved
for use in adults who have a body-mass index of 27 or higher plus
obesity-related medical conditions or a body-mass index of 30 or
higher in the absence of such conditions.7 In
1999, $321 million was spent in the United States on prescription
medications to treat obesity.8
Between 1996 and 1998, 2.5 percent of the adults in the United
States — or about 4.6 million persons — reported having used such
medications.9
Approximately 10 percent of women and 3 percent of men with a
body-mass index of 30 or higher have reported using weight-loss
medications for obesity.9 In
this review we briefly examine nonpharmacologic approaches to
promoting weight loss and give greater consideration to the use of
medications as adjunctive therapy in the management of obesity.
Nonpharmacologic Approaches to Weight Loss
Although 29 percent of the men in the United States and 44 percent
of the women describe themselves as trying to lose weight,10
only about 20 percent report restricting caloric intake and increasing
physical activity simultaneously, despite recommendations indicating
that this combination is effective.6
Many studies demonstrate that obese adults can lose about 0.5 kg per
week by decreasing their daily intake to 500 to 1000 kcal below the
caloric intake required for the maintenance of their current weight.11
More severe caloric restriction, with the use of diets that are very
low in calories, increases the rapidity of weight loss but not the
rate of long-term success in maintaining a reduced weight.6
Although adding exercise to caloric restriction minimally increases
weight loss during the acute phase of weight loss, it appears to be
the component of treatment that is most likely to promote long-term
maintenance of a reduced weight.12
Behavioral treatments help obese persons to develop adaptive thinking,
eating, and exercise habits that enable them to decrease their
weight and avoid regaining weight.11
Persons who combine caloric restriction and exercise with behavioral
treatment may expect to lose about 5 to 10 percent of
preintervention body weight over a period of four to six months.11
Although patients often perceive this "small" weight loss
as insufficient,13
it suffices to improve many obesity-related conditions.14
Unfortunately, improvements are not sustained if weight is
regained; and for the vast majority of persons, weight loss is
followed by a slow, inexorable climb to the preintervention body
weight — or even higher.15
Bariatric surgical treatments, such as gastric bypass, can induce
long-term weight loss, but are appropriate only for selected
patients with a body-mass index of at least 40 or a body-mass index
of at least 35 along with obesity-related medical conditions.6
Losing weight is difficult for most obese persons, yet long-term
maintenance of a reduced weight is even more challenging.
History of Pharmacotherapy for Obesity
For many years, obesity was approached as if it were either a
moral failing or evidence of underlying psychopathology.16
With the advent of behavioral treatments for obesity in the 1960s,17
hope arose that modification of maladaptive eating and exercise
habits would lead to sustained weight loss, and that time-limited
programs would produce permanent changes in weight. Medications for
the treatment of obesity were proposed as short-term adjuncts for
patients, who would presumably then acquire the skills necessary to
continue to lose weight, reach "ideal body weight," and
maintain a reduced weight indefinitely. Unfortunately, such
short-term approaches proved unsuccessful, and the history of many
ill-fated weight-loss regimens is well documented.18
The field underwent a paradigm shift in 1992 with the publication
of studies by Weintraub et al.19,20,21,22,23,24,25,26,27
concerning the efficacy of the combination of behavioral treatment
and two medications with different mechanisms of action — fenfluramine
and phentermine. Those studies found that weight loss could be
sustained for as long as three and a half years with continuing
pharmacotherapy. Although the number of patients studied was small,
the idea behind the approach — that obesity should be treated in the
same manner as any other chronic disease that might be ameliorated
through the long-term use of medication — differed dramatically from
those of prevailing approaches. The new approach meshed well with
the emerging realization that short-term treatments for obesity
generally do not lead to sustained weight reduction and that
patients are generally unwilling to continue behavioral treatment
indefinitely.11
Long-term use of weight-loss medications could be seen as a tool to
help patients adhere to the dietary and behavioral changes necessary
to maintain a reduced body weight.28,29
Although the subsequent withdrawal from the market of fenfluramine
(and dexfenfluramine) because of an association with valvular heart
disease30
had a dampening effect on the use of medications in the treatment
of obesity, an important lesson remained: obesity could and should
be approached as a chronic condition that requires continuing medical
care. For a minority of obese patients who have substantially increased
medical risk and for whom nonpharmacologic treatments alone prove
unsatisfactory, weight-loss medications may be useful adjuncts to
behavioral treatments.
Mechanisms of Action of Weight-Loss Drugs
Medications currently approved for weight loss in the United States
(Table 1)
fall into two broad categories: those that decrease food intake by
reducing appetite or increasing satiety (appetite suppressants) and
those that decrease nutrient absorption. A third category,
medications that increase energy expenditure, includes ephedrine,
which is not currently approved as a treatment for obesity in the
United States, and other investigational compounds.31
|
Appetite-Suppressant Medications
Most appetite suppressants work primarily by increasing the availability
of anorexigenic neurotransmitters — notably, norepinephrine,
serotonin, dopamine, or some combination of these neurotransmitters
— in the central nervous system.
Noradrenergic
Agents
Noradrenergic drugs available in the United States include
phentermine, diethylpropion, phendimetrazine, and benzphetamine (Table 1). Amphetamines
are no longer recommended (and are not approved for use) for weight
loss because of the potential for their abuse. Benzphetamine and
phendimetrazine, which are classified as Schedule III drugs by the
Drug Enforcement Administration (DEA), are considered by the DEA to
have substantially greater potential for abuse than those on
Schedule IV.
All of the above medications are approved by the Food and Drug
Administration (FDA) for use of "a few weeks" only (generally
presumed to be 12 weeks or less) for the treatment of obesity.7
Few studies of their safety and efficacy have extended to six months
or beyond.28
Such studies show a consistent but moderate difference in weight
loss (a difference of 2 to 10 kg) in comparisons with placebo.32,33,34,35,36,37
Side effects of noradrenergic medications include insomnia, dry mouth,
constipation, euphoria, palpitations, and hypertension.7
Although the most widely used of these compounds, phentermine, was
used in combination with fenfluramine, it has not been independently
associated with valvular heart disease.38
The only over-the-counter appetite-suppressant medication approved
for the treatment of obesity, phenylpropanolamine, was recently
withdrawn from the market because of concern about an association
with hemorrhagic stroke in women.39
Serotonergic
Agents
Serotonergic agents act by increasing the release of serotonin,
inhibiting its reuptake, or both. Fenfluramine (Pondimin) and dexfenfluramine
(Redux), medications that both stimulated serotonin release and
inhibited its reuptake, were withdrawn from the market in the United
States in 1997 because of associations with valvular heart disease
and pulmonary hypertension. Their efficacy in controlled studies
appeared similar to that of the noradrenergic agents.28,40
Selective serotonin-reuptake inhibitors are currently approved
for a number of indications that are not related to obesity, including
depression and obsessive–compulsive disorder. Some selective
serotonin-reuptake inhibitors have induced weight loss in short-term
studies, and fluoxetine (Prozac) (at a dose of 60 mg) has undergone
considerable evaluation to determine its efficacy for weight loss.41,42
Unfortunately, although patients who received fluoxetine for six
months lost more weight than those who received placebo, steady
regain occurred during the next six months despite the continuation
of medication, eroding any difference between the treatment groups.28
Sertraline (Zoloft), evaluated as an adjunct for weight maintenance
after a very-low-calorie diet, showed a similar lack of long-term
efficacy.43
Mixed
Noradrenergic–Serotonergic Agents
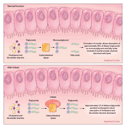
Sibutramine (Meridia), an inhibitor of both norepinephrine
reuptake and serotonin reuptake that also weakly inhibits dopamine
reuptake (Figure 1), is
approved by the FDA for weight loss and weight maintenance in conjunction
with a reduced-calorie diet.7
Sibutramine is given in a dose of 10 to 15 mg once daily and may be
given in a 5-mg dose to patients who do not tolerate the 10-mg dose.
Unlike fenfluramine and dexfenfluramine, it does not induce serotonin
release, and has not been implicated in the development of valvular
heart disease.44,45
Over a six-month period, subjects who follow a reduced-calorie diet
and receive sibutramine typically lose 5 to 8 percent of their
preintervention body weight, as compared with 1 to 4 percent among
subjects who receive placebo.46,47,48,49
Sibutramine-induced reductions in weight appear to be largely maintained
for periods of up to one year and remain significantly greater than
those observed in patients who receive placebo.50
Published studies with up to two years of data are now available. The
Sibutramine Trial of Obesity Reduction and Maintenance followed 605
European adults who took 10 mg of sibutramine daily for 6 months,
after which 467 participants who had lost more than 5 percent of
their preintervention body weight were randomly assigned to continue
to receive sibutramine or to receive placebo for 18 months.51
Although weight was regained in both groups during the second year
of follow-up, weight losses were significantly greater among those
who received sibutramine for the full two years of the trial. More
than 25 percent of those who continued to take sibutramine
maintained their reduced weight for the entire observation period.
As in most studies of weight-loss medications, the large numbers of
dropouts in both the study-drug group and the placebo group limit
the generalizability of the findings.51
By the end of the study, the dose of sibutramine had been increased
to 20 mg, a dose higher than is approved in the United States, in 52
percent of the subjects taking the medication. However, 86 percent
of the subjects who did not regain any of the weight they had lost
were taking no more than 15 mg of sibutramine daily. Sibutramine may
also increase weight loss and improve maintenance of reduced weight
in subjects who have previously lost weight with a very-low-calorie
diet.52
|
Side effects of sibutramine include increases in blood pressure and
pulse; although these are usually mild, they lead to the discontinuation
of sibutramine in up to 5 percent of patients.47,48,50,51
In general, reductions in blood pressure in those who lose weight with
sibutramine are less than the reductions in blood pressure seen with
similar weight loss obtained with other treatments.47
Adverse reactions also include dry mouth, headache, insomnia, and
constipation.45
Commensurate with weight loss, other metabolic risk factors improve;
these include hyperlipidemia and hyperuricemia, as well as glycemic
control and plasma insulin levels in patients with type 2 diabetes.46,48,50,51,53
Medications That Reduce Nutrient
Absorption
The only FDA-approved medication for obesity that reduces nutrient
absorption is orlistat (Xenical), which acts by binding to
gastrointestinal lipases in the lumen of the gut, preventing
hydrolysis of dietary fat (triglycerides) into absorbable free fatty
acids and monoacylglycerols (Figure 2).
Patients who take 120 mg of orlistat with or up to one hour after
meals excrete in the stool approximately one third of the dietary
fat they ingest, thereby reducing calorie and fat intake. In
double-blind, placebo-controlled trials, orlistat had moderate
efficacy for weight loss in adults. Orlistat-treated subjects who
completed trials lasting one year lost approximately 9 percent of
their preintervention body weight, as compared with 5.8 percent
among those who took placebo.54
|
Orlistat has also been found to slow the rate of regain of weight during
a second year of use; orlistat-treated subjects regained less weight
during the second year than placebo-treated subjects did (a regain
of 35.2 percent vs. 62.4 percent, a difference of about 2.5 kg).55,56,57
In these long-term studies, orlistat-treated patients also had
moderate decreases in diastolic blood pressure, insulin levels while
fasting, and total cholesterol and low-density lipoprotein
cholesterol, with a small cholesterol-lowering effect that was
independent of weight loss. Orlistat induced small reductions in
body weight in patients with type 2 diabetes that were nevertheless
significantly greater than those that occurred in such patients who
received placebo (losses of 6.2 kg vs. 4.3 kg); orlistat also led to
improvement in glycosylated hemoglobin values and a decreased
requirement for sulfonylurea drugs.58
Orlistat appears to have similar efficacy regardless of whether it
is prescribed in a primary care or a specialized treatment setting.59
Side effects of orlistat include flatulence with discharge, fecal
urgency, fecal incontinence, steatorrhea, oily spotting, and
increased frequency of defecation. These side effects are usually mild
to moderate, and generally decrease in frequency with ongoing
treatment. However, such side effects lead to discontinuation in
nearly 9 percent of patients, as compared with a rate of discontinuation
of 5 percent among patients treated with placebo.7,54
Orlistat also decreases absorption of fat-soluble vitamins, primarily
vitamin D, an effect that can be counteracted by daily administration
of a multivitamin at least two hours before or after a dose of
orlistat.7
Dietary Supplements and Herbal Preparations
Unlike prescription and over-the-counter medications, dietary
supplements are not prospectively reviewed for safety or efficacy by
the FDA, which takes action only if a dietary supplement is shown to
present "a significant or unreasonable risk."60
Producers of a dietary supplement cannot claim that it treats a
disease (including obesity) but may claim that it reduces the risk
of a disease in a population.60
Allison and colleagues61
critically reviewed the published literature on herbal and dietary
supplements for which claims have been made about the promotion of
weight loss: chitosan, chromium picolinate, conjugated linoleic
acid, ephedra alkaloids (ma huang), and garcinia cambogia. They
found that most such reports were based on poorly designed trials
that lacked randomization, blinding, or control groups. Although some
dietary supplements have mechanisms of action that could plausibly
lead to weight loss or have shown promising results in small-scale
studies in humans or animals, Allison et al. found that there were
insufficient data to provide evidence of either the safety or the
efficacy of any of these compounds as agents promoting weight loss.
Herbal compounds containing ephedra alkaloids and caffeine are the
only types for which there are data from randomized, double-blind, placebo-controlled
trials indicating efficacy in promoting weight loss.61,62,63
However, all such studies have been short-term (six months or less).
Ephedrine is an adrenergic agent with thermogenic and
appetite-suppressant properties.49
In the United States, ephedrine is approved for nonprescription use
for mild asthma and upper respiratory symptoms. Ephedrine in
combination with caffeine, aspirin, or both, has been found in
controlled trials to produce greater weight losses than placebo for
periods of up to one year, although most studies have been
short-term.31,64,65,66,67
Ma huang (Ephedra sinica) is
a botanical source of ephedra alkaloids often included in dietary
supplements sold for the purpose of promoting weight loss. Dietary
supplements containing ephedra alkaloids frequently contain a dosage
that differs substantially from that indicated on the product label.68
Case reports concerning ephedra alkaloids (often in combination with
caffeine) have noted serious cardiovascular and central nervous
system events, including hypertension, cardiac arrhythmia, stroke,
seizure, myocardial infarction, and sudden death.69
However, the incidence of such events among persons who take ephedra
alkaloids at the recommended doses has not been established.70
Because of the unpredictable amounts of active ingredients and the
potential for harmful effects, the National Institutes of Health
guidelines state that herbal preparations are not recommended as
part of a weight-loss program.6
Weight-Loss Medications Currently in Clinical
Trials
Medications Approved by the FDA for
Indications Other Than Obesity
A number of medications with different mechanisms of action are
currently in clinical trials to evaluate their safety and efficacy
in obese patients.
Bupropion (Wellbutrin) is an atypical antidepressant that is chemically
unrelated to tricyclic agents or selective serotonin-reuptake inhibitors.
It is a relatively weak inhibitor of the reuptake of norepinephrine,
serotonin, and dopamine and has structural similarities to
diethylpropion.7
After it was noted in studies concerning depression that bupropion
was associated with small weight losses, trials evaluating the use
of sustained-release bupropion in the treatment of obesity were
initiated and are currently being conducted.71,72
Bupropion is contraindicated in patients with seizure disorders.7
In several clinical trials studying the safety and efficacy of
topiramate (Topamax), a novel antiepileptic agent, for seizure control73
or affective disorders,74
reduced food intake and weight loss were noted. Trials of the safety
and efficacy of topiramate in obese patients who do not have
seizures, including patients with binge eating disorder75
or hypothalamic obesity,76
are ongoing. Adverse effects of topiramate include kidney stones and
central nervous system symptoms, such as paresthesias, dizziness, fatigue,
and somnolence.77
Metformin (Glucophage), a medication that inhibits hepatic glucose
production and improves sensitivity to insulin, is approved by
the FDA for the treatment of type 2 diabetes. Metformin not only
reduces hyperglycemia but has also been noted to prevent weight gain
or even to induce small weight losses in adults.78,79
Metformin is being studied in overweight, nondiabetic adults and
children to determine its effects on body weight, insulin resistance,
and related medical conditions.80,81,82
Metformin may cause nausea, flatulence, bloating, and diarrhea at
the beginning of treatment, and approximately 5 percent of adults
cannot take the drug at any dose because of such side effects.83
The most serious side effect of metformin is lactic acidosis, which
is estimated to occur at a rate of 3 cases per 100,000 patient-years
of exposure, primarily in patients with renal insufficiency,
congestive heart failure, pulmonary disease, or liver disease, which
are contraindications to its use.84
Investigational Medications
Levels of leptin, a hormone secreted by adipocytes, reflect the
lipid content of the total body of a nonfasting person.85
In a few children, severe, early-onset obesity has been associated with
an inability to produce functional leptin protein.86
Treatment of a leptin-deficient girl with recombinant human leptin
induced a dramatic reduction in body weight (a loss of 16.4 kg) and
changes in body composition.87
Less dramatic changes have been observed in adults with sufficient
leptin levels who are treated with recombinant human leptin. A
dose-dependent decrease in body weight and fat was observed, and the
greatest weight loss (a mean [±SD] loss of 7.1±8.5 kg) occurred in
eight persons who took leptin in a daily dose of 0.3 mg per kilogram
of body weight for 24 weeks.88
Pain and induration at the injection site, especially at higher
doses, led to discontinuation in some patients. Ongoing studies are
evaluating both different formulations of leptin89
and leptin-replacement therapy during low-calorie dieting.90
It remains unknown whether treatment with leptin will be beneficial
in persons without mutations in the leptin gene.
Other medications that are currently in clinical trials to
determine their ability to induce weight loss include ciliary
neurotrophic factor,91 a
neuroactive cytokine that exerts its effects through a receptor
whose mode of signal transduction is similar to that of the leptin
receptor; a peptide analogue of the human growth hormone fragment
177-19192;
and agonists of the ![]() 3-adrenergic
and cholecystokinin-A receptors.93
3-adrenergic
and cholecystokinin-A receptors.93
Strategies for Use of Medications in the
Treatment of Obesity
Because obesity is a chronic condition, pharmacotherapy should
be initiated with the expectation that long-term use will most likely
be needed.28
Numerous studies indicate that, just as blood pressure may increase
when antihypertensive drugs are discontinued, regaining of weight is
extremely likely when weight-loss medications are discontinued.28,52
Therefore, careful consideration of the known (and possible) risks
of long-term medical therapy must be weighed against potential
improvements in the patient's risk of obesity-related disease.
National Institutes of Health guidelines recommend that
pharmacotherapy should be initiated only in patients with a
body-mass index of at least 30 in the absence of obesity-related
medical conditions or a body-mass index of at least 27 in the
presence of such conditions.6
Approved prescription medications for weight loss appear to have
similar efficacy in controlled studies. Other than weight loss
during the first few weeks of drug treatment, no predictors of
responsiveness in an individual patient or class of patients have
been established. Therefore, an empirical choice of a specific medication
should be based on consideration of underlying medical conditions or
contraindications to particular drugs, concurrent medications, need
for monitoring, approval for long-term use, cost, and the preference
of the patient. National Institutes of Health guidelines suggest
that nonpharmacologic therapies should be attempted for six months
and that weight-loss drugs should be considered if weight loss is
unsatisfactory (e.g., less than 0.45 kg per month).94
In addition, behavioral treatment combined with pharmacotherapy may
result in better outcome than drug treatment alone.6,95
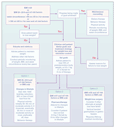
An evidence-based algorithm representing an overall approach to the
treatment of obesity is shown in Figure 3. A
practical guide designed to assist physicians in treating obese
patients in the primary care setting is available.94
|
Identification of Patients with a Response to Treatment
Although the mean weight loss attributable to medications for
obesity is less than 5 percent, individual patients do have more
robust responses. As compared with placebo, pharmacotherapy more
than doubles the percentage of patients in whom a weight loss of 5
or 10 percent is achieved.6,28
Identification of patients with a response would enable the risks
and costs of drug treatment to be concentrated among those who are
most likely to benefit. In addition to preintervention body weight,51 success
during the first month of therapy usually predicts ultimate weight
loss.6,28
Therefore, in patients without a weight loss of at least 2.0 kg
during the first four weeks of treatment, adherence to the
medication, diet, and exercise recommendations should be reassessed
and the possible need for an adjustment in the dosage should be
considered. If there continues to be minimal response to the
medication, the clinician should consider discontinuing it or
substituting another medication.28
For none of the currently available medications does the dose need
to be tapered before treatment is stopped.6
Pharmacotherapy for the Prevention of
Weight Regain
A major area of promise for pharmacotherapy is in enhancing weight
maintenance in those who have lost weight by a variety of methods.28
Because almost all nonsurgical obesity treatments lead to weight
loss for the first four to six months followed by regain,
pharmacotherapy can be instituted either to enhance weight loss
during the active weight-loss phase or to prevent later regain.
Although longer-term studies suggest that weight-loss medications
can decrease the rate of regain,51,52,56,57
it is important to note that data on the safety and effectiveness
of use for more than two years are not available for any FDA-approved
weight-loss medication.
Off-Label Use of FDA-Approved Medications
Use of FDA-approved medications in a manner inconsistent with
labeling is considered off-label use, a common practice in medicine.
Prudent physicians should inform patients whenever they prescribe medications
to be used in a manner that is not consistent with FDA labeling and
should discuss the paucity of data on safety and efficacy and obtain
the patient's consent for such use. Whenever possible, patients who
wish to use medications in such a manner should be encouraged to
participate in clinical trials.28
Intermittent
Use
Several small, short-term studies have described good results
with the intermittent use (e.g., in alternating months) of
appetite-suppressant weight-loss drugs.18
One fairly large study found that the safety and efficacy of
intermittent use of sibutramine (12 weeks of the drug alternating
with 12 weeks of placebo) were similar to those of continuous
sibutramine treatment.96
An increase in the rate of side effects just after drugs are stopped
or restarted has been noted in other studies.25,34
Further studies of the intermittent use of such medications would
help to answer questions about the effectiveness of this approach.
Drug
Combinations
Treatment in which drugs of different therapeutic classes are
combined in order to enhance efficacy or reduce adverse effects is
common in the treatment of chronic diseases. It is possible that
combination drug treatments for obesity will eventually be developed
that are both safe and effective. However, in the absence of data
from large trials, clinicians must exercise great caution in
recommending unproven therapeutic combinations. For the most part,
only case series97,98,99
and preliminary studies100,101,102
have examined the use of combinations of medications to enhance
weight loss. A study in which orlistat was added to sibutramine
treatment after one year of sibutramine alone found no enhancement
of weight loss.103
At present, using more than one drug in combination for the
treatment of obesity cannot be recommended outside clinical trials.6
Treatment
of Children and Adolescents
In general, the only children and adolescents who should be considered
for pharmacotherapy are those with a body-mass index in the 95th
percentile or higher for their age and sex plus an obesity-related
medical condition that may be remediable by a reduction of weight.
In selected populations of children 6 to 12 years old, intensive,
family-based behavioral treatment programs have been found to have a
favorable effect on children's weight for as long as 10 years.104
In contrast, there have been no long-term (i.e., at least one year),
randomized, double-blind, placebo-controlled trials involving
children (defined, for FDA regulatory purposes, as persons less than
16 years of age) that have demonstrated the safety and effectiveness
of weight-loss medications. The safety and effectiveness of orlistat
and sibutramine for children and adolescents have not been
established.7
Medications that are currently in clinical trials involving children
include orlistat,105
sibutramine,106
ephedrine–caffeine,107
and metformin.82
In addition, octreotide (a somatostatin agonist) is being evaluated
in children and adolescents with the hypothalamic obesity syndrome.108
Further studies are needed before pharmacotherapy outside clinical
trials can be recommended for younger patients.
Summary
Obesity is a serious and prevalent disorder whose effective management
requires ongoing care. Currently approved prescription medications
for weight loss, although moderate in their efficacy, can help
carefully selected obese patients lose weight and can reduce the
rate of regain. Behavioral interventions to improve diet and
increase physical activity are considered the primary means to
promote and maintain weight loss. Weight-loss medications should be
considered as an adjunct only for patients who are at substantial
medical risk because of their obesity and in whom nonpharmacologic
treatments have not resulted in sufficient weight loss to improve
health or to prevent regain. The safety and efficacy of weight-loss
medications beyond two years of use have not been established. In
addition, although some risk factors for obesity-related disease are
improved with the use of weight-loss medications, the long-term
effect of such medications on morbidity and mortality has not been
determined.
In many ways, the current state of treatment for obesity is similar
to the state of the treatment of hypertension several decades ago.18
Few medications were available, their efficacy was limited, and
predictors of response were lacking. Just as research into the
underlying causes and consequences of hypertension has led to
dramatic improvements in its treatment, advances in our
understanding of energy balance will most likely lead to more
effective treatments for obesity in the future. With such
understanding, we can hope not only to develop safe and effective
ways to help obese persons to achieve and maintain a healthy weight
but also to understand how to prevent the development of obesity in
those who are at risk.
Supported
by a grant (ZO1 HD-00641) from the National Institute of Child
Health and Human Development (to Dr. Jack A. Yanovski). The opinions
expressed herein are those of the authors and do not necessarily
reflect the views of the National Institutes of Health, the Public
Health Service, or the Department of Health and Human Services.
Source Information
From the Division of Digestive Diseases and Nutrition, National
Institute of Diabetes and Digestive and Kidney Diseases (S.Z.Y.) and the Unit
on Growth and Obesity, Developmental Endocrinology Branch, National Institute
of Child Health and Human Development (J.A.Y.), National Institutes of Health,
Bethesda, Md.
Address reprint requests to Dr. Susan Yanovski at the Obesity and
Eating Disorders Program, NIDDK, 6707 Democracy Blvd., Bethesda, MD 20892-5450,
or at [log in to unmask].
References
- Prevalence
of overweight and obesity among adults: United States, 1999. Hyattsville,
Md.: National Center for Health Statistics, Health E-Stats, 2000.
(Accessed December 7, 2001, at http://www.cdc.gov/nchs/products/pubs/pubd/hestats/obese/obse99.htm.)
- Flegal
KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the
United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab
Disord 1998;22:39-47.[Medline]
- Troiano
RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight
prevalence and trends for children and adolescents: the National Health
and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med
1995;149:1085-1091.[Medline]
- From the
Centers for Disease Control and Prevention: update: prevalence of overweight
among children, adolescents, and adults -- United States, 1988-1994. JAMA
1997;277:1111-1111.[Medline]
- National
Task Force on the Prevention and Treatment of Obesity. Overweight,
obesity, and health risk. Arch Intern Med 2000;160:898-904.[Medline]
- Clinical
guidelines on the identification, evaluation, and treatment of overweight
and obesity in adults -- the Evidence Report. Obes Res 1998;6:Suppl
2:51S-209S.[Medline]
- Physicians'
desk reference. 55th ed. Montvale, N.J.: Medical Economics, 2001.
- Wilhelm
C. Growing the market for anti-obesity drugs. Chemical Market Reporter.
May 15, 2000:FR23-FR24.
- Khan LK,
Serdula MK, Bowman BA, Williamson DF. Use of prescription weight loss
pills among U.S. adults in 1996-1998. Ann Intern Med 2001;134:282-286.[Medline]
- Serdula
MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW.
Prevalence of attempting weight loss and strategies for controlling
weight. JAMA 1999;282:1353-1358.[Medline]
- Wadden
TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am
2000;84:441-461.[Medline]
- McGuire
MT, Wing RR, Klem ML, Hill JO. Behavioral strategies of individuals who
have maintained long-term weight losses. Obes Res 1999;7:334-341.[Abstract]
- Foster
GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss?
Patients' expectations and evaluations of obesity treatment outcomes. J
Consult Clin Psychol 1997;65:79-85.[Medline]
- Blackburn
G. Effect of degree of weight loss on health benefits. Obes Res
1995;3:Suppl 2:211s-216s.[Medline]
- NIH
Technology Assessment Conference Panel. Methods for voluntary weight loss
and control. Ann Intern Med 1993;119:764-770.[Medline]
- Bray GA.
Historical framework for the development of ideas about obesity. In: Bray
GA, Bouchard C, James WPT, eds. Handbook of obesity. New York: Marcel
Dekker, 1998:1-29.
- Stuart
RB. Behavioral control of overeating: 1967. Obes Res 1996;4:411-417.[Medline]
- Bray GA,
Greenway FL. Current and potential drugs for treatment of obesity. Endocr
Rev 1999;20:805-875.[Abstract/Full
Text]
- Weintraub
M. Long-term weight control study: conclusions. Clin Pharmacol Ther
1992;51:642-646.[Medline]
- Weintraub
M, Sundaresan PR, Schuster B. Long-term weight control study. VII (weeks 0
to 210). Serum lipid changes. Clin Pharmacol Ther 1992;51:634-641.[Medline]
- Weintraub
M, Sundaresan PR, Cox C. Long-term weight control study. VI. Individual
participant response patterns. Clin Pharmacol Ther 1992;51:619-633.[Medline]
- Weintraub
M, Sundaresan PR, Schuster B, Averbuch M, Stein EC, Byrne L. Long-term
weight control study. V (weeks 190 to 210). Follow-up of participants
after cessation of medication. Clin Pharmacol Ther 1992;51:615-618.[Medline]
- Weintraub
M, Sundaresan PR, Schuster B, et al. Long-term weight control study. IV
(weeks 156 to 190). The second double-blind phase. Clin Pharmacol Ther
1992;51:608-614.[Medline]
- Weintraub
M, Sundaresan PR, Schuster B, Moscucci M, Stein EC. Long-term weight
control study. III (weeks 104 to 156). An open-label study of dose
adjustment of fenfluramine and phentermine. Clin Pharmacol Ther
1992;51:602-607.[Medline]
- Weintraub
M, Sundaresan PR, Schuster B, et al. Long-term weight control study. II
(weeks 34 to 104). An open-label study of continuous fenfluramine plus
phentermine versus targeted intermittent medication as adjuncts to
behavior modification, caloric restriction, and exercise. Clin Pharmacol
Ther 1992;51:595-601.[Medline]
- Weintraub
M, Sundaresan PR, Madan M, et al. Long-term weight control study. I (weeks
0 to 34). The enhancement of behavior modification, caloric restriction,
and exercise by fenfluramine plus phentermine versus placebo. Clin
Pharmacol Ther 1992;51:586-594.[Medline]
- Weintraub
M. Long-term weight control: the National Heart, Lung, and Blood Institute
funded multimodal intervention study. Clin Pharmacol Ther 1992;51:581-585.
[Erratum, Clin Pharmacol Ther 1992;52:323.][Medline]
- National
Task Force on the Prevention and Treatment of Obesity. Long-term
pharmacotherapy in the management of obesity. JAMA 1996;276:1907-1915.[Medline]
- Stallone
DD, Stunkard AJ. Long-term use of appetite suppressant medication:
rationale and recommendations. Drug Dev Res 1992;26:1-20.
- Connolly
HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with
fenfluramine-phentermine. N Engl J Med 1997;337:581-588. [Erratum, N Engl
J Med 1997;337:1783.][Abstract/Full
Text]
- Astrup
A. Thermogenic drugs as a strategy for treatment of obesity. Endocrine
2000;13:207-212.[Medline]
- Inoue S.
Clinical studies with mazindol. Obes Res 1995;3:Suppl 4:549S-552S.[Abstract]
- Enzi G,
Baritussio A, Marchiori E, Crepaldi G. Short-term and long-term clinical
evaluation of a non-amphetaminic anorexiant (mazindol) in the treatment of
obesity. J Int Med Res 1976;4:305-318.[Medline]
- Steel
JM, Munro JF, Duncan LJ. A comparative trial of different regimens of
fenfluramine and phentermine in obesity. Practitioner 1973;211:232-236.[Medline]
- McKay
RH. Long-term use of diethylpropion in obesity. Curr Med Res Opin
1973;1:489-493.[Medline]
- Munro
JF, MacCuish AC, Wilson EM, Duncan LJP. Comparison of continuous and
intermittent anorectic therapy in obesity. BMJ 1968;1:352-354.
- Silverstone
JT, Solomon T. The long-term management of obesity in general practice. Br
J Clin Pract 1965;19:395-398.
- Connolly
HM, McGoon MD. Obesity drugs and the heart. Curr Probl Cardiol
1999;24:745-792.[Medline]
- Kernan
WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of
hemorrhagic stroke. N Engl J Med 2000;343:1826-1832.[Abstract/Full
Text]
- Guy-Grand
B. Clinical studies with dexfenfluramine: from past to future. Obes Res
1995;3:Suppl 4:491S-496S.[Abstract]
- Goldstein
DJ, Rampey AH, Dornseif BE, Levine LR, Potvin JH, Fludzinski LA.
Fluoxetine: a randomized clinical trial in the maintenance of weight loss.
Obes Res 1993;2:92-98.
- Goldstein
DJ, Rampey AH Jr, Enas GG, Potvin JH, Fludzinski LA, Levine LR.
Fluoxetine: a randomized clinical trial in the treatment of obesity. Int J
Obes Relat Metab Disord 1994;18:129-135.[Medline]
- Wadden
TA, Bartlett SJ, Foster GD, et al. Sertraline and relapse prevention
training following treatment by very-low-calorie diet: a controlled
clinical trial. Obes Res 1995;3:549-557.[Abstract]
- Bach DS,
Rissanen AM, Mendel CM, et al. Absence of cardiac valve dysfunction in
obese patients treated with sibutramine. Obes Res 1999;7:363-369.[Abstract]
- Sibutramine
for obesity. Med Lett Drugs Ther 1998;40:32-32.[Medline]
- Fanghanel
G, Cortinas L, Sanchez-Reyes L, Berber A. A clinical trial of the use of
sibutramine for the treatment of patients suffering essential obesity. Int
J Obes Relat Metab Disord 2000;24:144-150.[Medline]
- Bray GA,
Blackburn GL, Ferguson JM, et al. Sibutramine produces dose-related weight
loss. Obes Res 1999;7:189-198.[Abstract]
- Fujioka
K, Seaton TB, Rowe E, et al. Weight loss with sibutramine improves
glycaemic control and other metabolic parameters in obese patients with
type 2 diabetes mellitus. Diabetes Obes Metab 2000;2:175-187.[Medline]
- Ryan DH.
Use of sibutramine and other noradrenergic and serotonergic drugs in the
management of obesity. Endocrine 2000;13:193-199.[Medline]
- McMahon
FG, Fujioka K, Singh BN, et al. Efficacy and safety of sibutramine in
obese white and African American patients with hypertension: a 1-year,
double-blind, placebo-controlled, multicenter trial. Arch Intern Med
2000;160:2185-2191.[Medline]
- James
WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance
after weight loss: a randomised trial. Lancet 2000;356:2119-2125.[Medline]
- Apfelbaum
M, Vague P, Ziegler O, Hanotin C, Thomas F, Leutenegger E. Long-term
maintenance of weight loss after a very-low-calorie diet: a randomized
blinded trial of the efficacy and tolerability of sibutramine. Am J Med
1999;106:179-184.[Medline]
- Finer N,
Bloom SR, Frost GS, Banks LM, Griffiths J. Sibutramine is effective for
weight loss and diabetic control in obesity with type 2 diabetes: a
randomised, double-blind, placebo-controlled study. Diabetes Obes Metab
2000;2:105-112.[Medline]
- Heck AM,
Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management
of obesity. Pharmacotherapy 2000;20:270-279.[Medline]
- Davidson
MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor
reduction in obese subjects treated for 2 years with orlistat: a
randomized controlled trial. JAMA 1999;281:235-242. [Erratum, JAMA
1999;281:1174.][Medline]
- Hill JO,
Hauptman J, Anderson JW, et al. Orlistat, a lipase inhibitor, for weight
maintenance after conventional dieting: a 1-y study. Am J Clin Nutr
1999;69:1108-1116.[Abstract/Full
Text]
- Sjostrom
L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of
orlistat for weight loss and prevention of weight regain in obese
patients. Lancet 1998;352:167-172.[Medline]
- Hollander
PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of
obese patients with type 2 diabetes: a 1-year randomized double-blind
study. Diabetes Care 1998;21:1288-1294.[Abstract]
- Hauptman
J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term
treatment of obesity in primary care settings. Arch Fam Med 2000;9:160-167.[Medline]
- Lewis
CJ. Regulatory environment for dietary supplements and botanicals targeted
to weight loss. Crit Rev Food Sci Nutr 2001;41:43-44.[Medline]
- Allison
DB, Fontaine KR, Heshka S, Mentore JL, Heymsfield SB. Alternative
treatments for weight loss: a critical review. Crit Rev Food Sci Nutr
2001;41:1-28.[Medline]
- Boozer
CN, Daly PA, Blanchard D, Nasser JA, Solomon JL, Homel P. Herbal
ephedra/caffeine for weight loss: a 6-month safety and efficacy trial.
Obes Res 2001;9:68-68.abstract
- Boozer
CN, Nasser JA, Heymsfield SB, Wang V, Chen G, Solomon JL. An herbal
supplement containing Ma Huang-Guarana for weight loss: a randomized,
double-blind trial. Int J Obes 2001;25:316-324.
- Astrup
A, Breum L, Toubro S, Hein P, Quaade F. The effect and safety of an
ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in
obese subjects on an energy restricted diet: a double blind trial. Int J
Obes Relat Metab Disord 1992;16:269-277.[Medline]
- Breum L,
Pedersen JK, Ahlstrom F, Frimodt-Moller J. Comparison of an
ephedrine/caffeine combination and dexfenfluramine in the treatment of obesity:
a double-blind multi-centre trial in general practice. Int J Obes Relat
Metab Disord 1994;18:99-103.[Medline]
- Daly PA,
Krieger DR, Dulloo AG, Young JB, Landsberg L. Ephedrine, caffeine and
aspirin: safety and efficacy for treatment of human obesity. Int J Obes
Relat Metab Disord 1993;17:Suppl 1:S73-S78.
- Toubro
S, Astrup AV, Breum L, Quaade F. Safety and efficacy of long-term
treatment with ephedrine, caffeine and an ephedrine/caffeine mixture. Int
J Obes Relat Metab Disord 1993;17:Suppl 1:S69-S72.
- Gurley
BJ, Gardner SF, Hubbard MA. Content versus label claims in
ephedra-containing dietary supplements. Am J Health Syst Pharm
2000;57:963-969.[Medline]
- Haller
CA, Benowitz NL. Adverse cardiovascular and central nervous system events
associated with dietary supplements containing ephedra alkaloids. N Engl J
Med 2000;343:1833-1838.[Abstract/Full
Text]
- Fleming
GA. The FDA, regulation, and the risk of stroke. N Engl J Med 2000;343:1886-1887.[Full
Text]
- Gadde
KM, Krishnan KRR, Drezner MK. Bupropion SR shows promise as an effective
obesity treatment. Obes Res 1999;7:Suppl 1:51S-51S.abstract
- Anderson
JW, Greenway F, Fujioka K, Gadde K, McKenny J, O'Neil P. Clinical trial
using bupropion SR with a moderate-intensity lifestyle intervention. Obes
Res 2000;8:Suppl 1:88S-88S.abstract
- Smith U,
Axelsen M, Hellebö-Johanson E, Lundgren B, Ben-Menachem E. Topiramate, a
novel antiepileptic drug, reduces body weight and food intake in obesity.
Obes Res 2000;8:Suppl 1:10S-10S.abstract
- Teter
CJ, Early JJ, Gibbs CM. Treatment of affective disorder and obesity with
topiramate. Ann Pharmacother 2000;34:1262-1265.[Medline]
- Shapira
NA, Goldsmith TD, McElroy SL. Treatment of binge-eating disorder with
topiramate: a clinical case series. J Clin Psychiatry 2000;61:368-372.[Medline]
- Pennington
Biomedical Research Center scientific report: 2000. Baton Rouge, La.:
Pennington Biomedical Research Center, 2001.
- Glauser
TA. Topiramate. Epilepsia 1999;40:Suppl 5:S71-S80.
- Fontbonne
A, Charles MA, Juhan-Vague I, et al. The effect of metformin on the
metabolic abnormalities associated with upper-body fat distribution.
Diabetes Care 1996;19:920-926.[Abstract]
- DeFronzo
RA, Goodman AM, Multicenter Metformin Study Group. Efficacy of metformin
in patients with non-insulin-dependent diabetes mellitus. N Engl J Med
1995;333:541-549.[Abstract/Full
Text]
- The
Diabetes Prevention Program: baseline characteristics of the randomized
cohort. Diabetes Care 2000;23:1619-1629.[Abstract]
- Pasquali
R, Gambineri A, Biscotti D, et al. Effect of long-term treatment with
metformin added to hypocaloric diet on body composition, fat distribution,
and androgen and insulin levels in abdominally obese women with and
without the polycystic ovary syndrome. J Clin Endocrinol Metab
2000;85:2767-2774.[Abstract/Full
Text]
- Freemark
M, Bursey D. The effects of metformin on body mass index and glucose
tolerance in obese adolescents with fasting hyperinsulinemia and a family
history of type 2 diabetes. Pediatrics 2001;107:763-763.abstract
- Bailey CJ.
Biguanides and NIDDM. Diabetes Care 1992;15:755-772.[Abstract]
- Bailey
CJ, Turner RC. Metformin. N Engl J Med 1996;334:574-579.[Full
Text]
- Halaas
JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma
protein encoded by the obese gene. Science 1995;269:543-546.[Medline]
- Montague
CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is
associated with severe early-onset obesity in humans. Nature
1997;387:903-908.[Medline]
- Farooqi
IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a
child with congenital leptin deficiency. N Engl J Med 1999;341:879-884.[Full
Text]
- Heymsfield
SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in
obese and lean adults: a randomized, controlled, dose-escalation trial.
JAMA 1999;282:1568-1575.[Medline]
- Hukshorn
CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA.
Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB)
administration in obese men. J Clin Endocrinol Metab 2000;85:4003-4009.[Abstract/Full
Text]
- New
leptin trial begins. Obesity Meds and Research News. No. 6. June 2001.
(Alexandria, Va.: OMR.)
- Guler
HP, Ettinger MP, Littlejohn TW, et al. Axokine causes significant weight
loss in severely and morbidly obese subjects. Int J Obes Relat Metab
Disord 2001;25:Suppl 2:S111-S111.abstract
- Heffernan
MA, Jiang WJ, Thorburn AW, Ng FM. Effects of oral administration of a
synthetic fragment of human growth hormone on lipid metabolism. Am J
Physiol Endocrinol Metab 2000;279:E501-E507.[Medline]
- Drug
development update. Obesity Meds and Research News. No. 3. March 2001.
(Alexandria, Va.: OMR.)
- The
practical guide: identification, evaluation, and treatment of overweight
and obesity in adults. Bethesda, Md.: National Heart, Lung, and Blood
Institute, North American Association for the Study of Obesity, 2000. (NIH
publication no. 00-4048.) (Also available at http://www.nhlbi.nih.gov/guidelines/obesity/practgde.htm.)
- Wadden
TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of
lifestyle modification in the pharmacologic treatment of obesity: a
randomized trial. Arch Intern Med 2001;161:218-227.[Medline]
- Wirth A,
Krause J. Long-term weight loss with sibutramine: a randomized controlled
trial. JAMA 2001;286:1331-1339.[Medline]
- Griffen
L, Anchors M. The "phen-pro" diet drug combination is not
associated with valvular heart disease. Arch Intern Med 1998;158:1278-1279.[Medline]
- Atkinson
RL, Blank RC, Schumacher D, Dhurandhar NV, Ritch DL. Long-term drug
treatment of obesity in a private practice setting. Obes Res
1997;5:578-586.[Abstract]
- Bowen
RL, Atkinson RL. Addition of orlistat to long term phentermine treatment
for obesity. Obes Res 2000;8:118-118.abstract
- Devlin
MJ, Goldfein JA, Carino JS, Wolk SL. Open treatment of overweight binge
eaters with phentermine and fluoxetine as an adjunct to
cognitive-behavioral therapy. Int J Eat Disord 2000;28:325-332.[Medline]
- Alger
SA, Malone M, Cerulli J, Fein S, Howard L. Beneficial effects of
pharmacotherapy on weight loss, depressive symptoms, and eating patterns
in obese binge eaters and non-binge eaters. Obes Res 1999;7:469-476.[Abstract]
- Dhurandhar
NV, Atkinson RL. Comparison of serotonin agonists in combination with
phentermine for treatment of obesity. FASEB J 1996;10:A561-A561.abstract
- Wadden
TA, Berkowitz RI, Womble LG, Sarwer DB, Arnold ME, Steinberg CM. Effects
of sibutramine plus orlistat in obese women following 1 year of treatment
by sibutramine alone: a placebo-controlled trial. Obes Res 2000;8:431-437.[Abstract/Full
Text]
- Epstein
LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral
family-based treatment for childhood obesity. Health Psychol
1994;13:373-383.[Medline]
- McCann
S, McDuffie J, Nicholson J, Sastry L, Calis K, Yanovski J. A pilot study
of the efficacy of orlistat in overweight adolescents. Obes Res
2000;8:Suppl 1:58S-58S.abstract
- Roan S.
Are drugs the answer to childhood obesity? Los Angeles Times. December 25,
2000:S1.
- Molnar
D, Torok K, Erhardt E, Jeges S. Safety and efficacy of treatment with an
ephedrine/caffeine mixture: the first double-blind placebo-controlled
pilot study in adolescents. Int J Obes Relat Metab Disord
2000;24:1573-1578.[Medline]
- Lustig
RH, Rose SR, Burghen GA, et al. Hypothalamic obesity caused by cranial
insult in children: altered glucose and insulin dynamics and reversal by a
somatostatin agonist. J Pediatr 1999;135:162-168.[Medline]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.