|
|
Mild Therapeutic Hypothermia to Improve the
Neurologic Outcome after Cardiac Arrest
The Hypothermia after Cardiac Arrest Study Group
ABSTRACT
Background Cardiac
arrest with widespread cerebral ischemia frequently leads to severe
neurologic impairment. We studied whether mild systemic hypothermia
increases the rate of neurologic recovery after resuscitation from
cardiac arrest due to ventricular fibrillation.
Methods In this
multicenter trial with blinded assessment of the outcome, patients
who had been resuscitated after cardiac arrest due to ventricular
fibrillation were randomly assigned to undergo therapeutic
hypothermia (target temperature, 32°C to 34°C, measured in the
bladder) over a period of 24 hours or to receive standard treatment
with normothermia. The primary end point was a favorable neurologic
outcome within six months after cardiac arrest; secondary end points
were mortality within six months and the rate of complications
within seven days.
Results Seventy-five
of the 136 patients in the hypothermia group for whom data were
available (55 percent) had a favorable neurologic outcome
(cerebral-performance category, 1 [good recovery] or 2 [moderate
disability]), as compared with 54 of 137 (39 percent) in the
normothermia group (risk ratio, 1.40; 95 percent confidence
interval, 1.08 to 1.81). Mortality at six months was 41 percent in
the hypothermia group (56 of 137 patients died), as compared with 55
percent in the normothermia group (76 of 138 patients; risk ratio,
0.74; 95 percent confidence interval, 0.58 to 0.95). The
complication rate did not differ significantly between the two
groups.
Conclusions In patients
who have been successfully resuscitated after cardiac arrest due to
ventricular fibrillation, therapeutic mild hypothermia increased the
rate of a favorable neurologic outcome and reduced mortality.
An estimated 375,000 people in Europe
undergo sudden cardiac arrest yearly.1
Recovery without residual neurologic damage after cardiac arrest
with global cerebral ischemia is rare. After cardiac arrest with no
blood flow for more than five minutes, the generation of free
radicals, together with other mediators, during reperfusion creates
chemical cascades that result in cerebral injury.2
Until recently, there was no therapy with documented efficacy in
preventing brain damage after cardiac arrest.
Several studies have shown that moderate systemic hypothermia
(30°C)3
or mild hypothermia (34°C)4,5,6,7,8
markedly mitigates brain damage after cardiac arrest in dogs. The
exact mechanism for this cerebral resuscitative effect is not clear.
A reduction in cerebral oxygen consumption9,10 and
other multifactorial chemical and physical mechanisms during and
after ischemia have been postulated.11,12,13,14,15,16
These include retardation of destructive enzymatic reactions,
suppression of free-radical reactions, protection of the fluidity of
lipoprotein membranes, reduction of the oxygen demand in low-flow
regions, reduction of intracellular acidosis, and inhibition of the
biosynthesis, release, and uptake of excitatory neurotransmitters.
Preliminary clinical studies have shown that patients treated
with mild hypothermia after cardiac arrest have an improved neurologic
outcome, without important side effects, as compared with the
outcome in historical controls.17,18,19,20
We compared mild hypothermia with standard normothermia in
patients who had had cardiac arrest due to ventricular fibrillation.
The primary end point was a favorable neurologic outcome within six
months after cardiac arrest.21,22,23
Secondary end points were mortality at six months and the incidence
of complications during the first seven days. Nine centers in five
European countries participated in the study.
Methods
Patients
Patients seen consecutively in the emergency department in whom
spontaneous circulation had been restored after cardiac arrest were
eligible for the study. The criteria for inclusion were a witnessed
cardiac arrest, ventricular fibrillation or nonperfusing ventricular
tachycardia as the initial cardiac rhythm, a presumed cardiac origin
of the arrest, an age of 18 to 75 years, an estimated interval of 5
to 15 minutes from the patient's collapse to the first attempt at resuscitation
by emergency medical personnel, and an interval of no more than 60
minutes from collapse to restoration of spontaneous circulation.
Patients were excluded if they met any of the following criteria:
a tympanic-membrane temperature below 30°C on admission, a
comatose state before the cardiac arrest due to the administration of
drugs that depress the central nervous system, pregnancy, response
to verbal commands after the return of spontaneous circulation and
before randomization, evidence of hypotension (mean arterial
pressure, less than 60 mm Hg) for more than 30 minutes after the
return of spontaneous circulation and before randomization, evidence
of hypoxemia (arterial oxygen saturation, less than 85 percent) for more
than 15 minutes after the return of spontaneous circulation and
before randomization, a terminal illness that preceded the arrest,
factors that made participation in follow-up unlikely, enrollment in
another study, the occurrence of cardiac arrest after the arrival of
emergency medical personnel, or a known preexisting coagulopathy.
Study Design
The study was designed as a randomized, controlled trial with
blinded assessment of the outcome. The protocol and consent procedure
were approved by the institutional review board of each
participating center. For all patients, the requirement of informed
consent was waived in accordance with the ethical standards of the
local institutional review board and the guidelines for good
clinical practice of the European Agency for the Evaluation of
Medicinal Products.24
The patient's family was informed about the trial, and the protocol
specified that if there were any objections, the patient would be
withdrawn from the study. However, there were no objections.
Treatment assignments were randomly generated by computer in blocks
of 10, with stratification according to center. Sealed envelopes
containing the treatment assignments were provided by the
biostatistics center. Immediately after a patient had been enrolled,
an envelope was opened, and the patient was assigned to the
specified group.
Personnel involved in the care of patients during the first 48
hours after cardiac arrest could not be blinded with respect to
treatment assignments. However, the physicians responsible for
assessing the neurologic outcome within the first six months after
the arrest were unaware of the treatment assignments.
Treatment
All patients received standard intensive care according to a detailed
protocol. Sedation was induced by the intravenous administration of
midazolam (0.125 mg per kilogram of body weight per hour initially)
and fentanyl (0.002 mg per kilogram per hour initially), and the
doses were adjusted as needed for 32 hours for the management of
mechanical ventilation. To prevent shivering, paralysis was induced
by the intravenous administration of pancuronium (0.1 mg per
kilogram) every 2 hours for a total of 32 hours. Intracranial pressure
was not monitored.
The temperature on admission was measured with an infrared
tympanic thermometer (Ototemp LighTouch, Exergen, Watertown, Mass.).
Further temperature measurements were made with a bladder-temperature
probe (Foley catheter). Patients randomly assigned to the normothermia
group were placed on a conventional hospital bed, and normothermia was
maintained. Those randomly assigned to the hypothermia group were
cooled to a target temperature of 32°C to 34°C with the use of an
external cooling device (TheraKool, Kinetic Concepts, Wareham,
United Kingdom). This device consists of a mattress with a cover
that delivers cold air over the entire body. The goal was to reach
the target bladder temperature within four hours after the return of
spontaneous circulation. If this goal was not achieved, ice packs
were applied. The temperature was maintained at 32°C to 34°C for 24
hours from the start of cooling, followed by passive rewarming,
which we expected would occur over a period of 8 hours.
Data Collection
Data on cardiac arrest for individual patients were recorded in
the Utstein style.25
Laboratory tests were performed at base line, 12 and 48 hours after
cardiac arrest, and as clinically indicated. Risk factors for an
unfavorable outcome (hypotension or a nonfatal cardiac arrest after
resuscitation) were documented.
Outcome
The primary outcome was a favorable neurologic outcome within
six months, defined as a Pittsburgh cerebral-performance category of
1 (good recovery) or 2 (moderate disability) on a five-category scale;
the other categories were 3 (severe disability), 4 (a vegetative
state), and 5 (death).21,22,23
The neurologic outcome was determined without knowledge of the patient's
treatment assignment. Patients with good recovery or moderate
disability had sufficient cerebral function to live independently
and work at least part-time.
Secondary end points were overall mortality at six months and
the rate of complications during the first seven days after cardiac
arrest. Bleeding of any severity, pneumonia, sepsis, pancreatitis,
renal failure, pulmonary edema, seizures, arrhythmias, and pressure
sores were recorded. Since an individual patient might have more
than one complication at a time, the occurrence of at least one
complication of any kind per patient was also documented.
Statistical Analysis
Continuous variables, which were not normally distributed, are
reported as medians and interquartile ranges. Categorical variables are
reported as counts and percentages. Primary and secondary outcomes
were binary, and the chi-square test or Fisher's exact test, as
appropriate, was used to compare outcomes in the hypothermia and
normothermia groups. Trends across subgroups were measured with an
extension of the Wilcoxon rank-sum test.26
The difference in risk between the two groups, with the
corresponding 95 percent confidence interval, was calculated as a
measure of the absolute risk, which was then used to calculate the
number needed to treat. Risk ratios are reported as a measure of
relative risk.
We used a multivariate logistic-regression model to determine
whether the association between the intervention and the primary and
secondary outcomes (neurologic recovery and mortality) was confounded
by base-line differences between the study groups. All the
covariables listed in Table 1 were
entered into the model, except for the dose of epinephrine, which
was excluded because of collinearity with the interval from the
patient's collapse to the restoration of spontaneous circulation. We
converted odds ratios to risk ratios using the following formula:
|
risk ratio = odds ratio ÷ ([1 – incidence in normothermia group] +
incidence in normothermia group x odds ratio).27
Confounding can be assumed if the crude risk ratio differs from
the adjusted risk ratio. Goodness of fit was assessed with the Hosmer–Lemeshow
chi-square test. A reasonable fit can be assumed if the result is
not significant at the 5 percent level. Analysis was carried out
according to the intention-to-treat principle. Stata software
(version 7, Stata, College Station, Tex.) was used to analyze the
data.
Results
The study was carried out between March 1996 and January 2001.
Since the enrollment rate was lower than expected and funding had
ended by July 2000, enrollment was stopped at this date.
A total of 3551 patients were assessed for eligibility; 3246 of
these patients did not meet the inclusion criteria, and 30 were not
included because of logistic problems. Thus, 275 patients were
enrolled, with 137 patients randomly assigned to the hypothermia group
and 138 to the normothermia group (i.e., the group that received
standard care after resuscitation). Hypothermia was discontinued
early in 14 patients for the following reasons: death (6 patients),
arrhythmia and hemodynamic instability (3), technical problems with
the cooling device (2), liver rupture (1), previous random
assignment to the hypothermia group (1), and an error in the
duration of cooling (1). All randomized patients were included in
the analysis of mortality. One patient in each group was lost to
follow-up for neurologic status.
At base line, the patients in the two groups were generally similar,
although the patients in the normothermia group were more likely to
have a history of diabetes mellitus or coronary heart disease and to
have received basic life support from a bystander than were those in
the hypothermia group. These differences appear to have been due to
random variation (Table
1).
Cooling
In patients randomly assigned to the hypothermia group, the median
interval between the restoration of spontaneous circulation and the
initiation of cooling was 105 minutes (interquartile range, 61 to
192). The median interval between the restoration of spontaneous
circulation and the attainment of a temperature between 32°C and
34°C was 8 hours (interquartile range, 4 to 16). In 19 patients, the
target temperature could not be reached. Ice packs were required for
93 of the 132 patients for whom data were available (70 percent).
The median duration of cooling was 24 hours (interquartile range, 24
to 25), and among patients in whom the target temperature was
reached, it was maintained for a median of 24 hours (interquartile
range, 12 to 29). Passive rewarming to a temperature above 36°C
lasted for a median of 8 hours (interquartile range, 8 to 12). The
temperature curves for the normothermia and hypothermia groups are
shown in Figure 1.
|
Outcome at Six Months
A total of 75 of the 136 patients (55 percent) in the hypothermia
group had a favorable neurologic outcome, as compared with 54 of
the 137 (39 percent) in the normothermia group (risk ratio, 1.40; 95
percent confidence interval, 1.08 to 1.81) (Table 2). To
prevent one unfavorable neurologic outcome, 6 patients would need to
be treated with hypothermia (95 percent confidence interval, 4 to 25
patients). After adjustment for a history of diabetes mellitus, a
history of coronary heart disease, and receipt of basic life support
from a bystander, the risk ratio changed only marginally (data not
shown). After adjustment for all the base-line variables shown in Table 1, the risk
ratio increased slightly, to 1.47 (95 percent confidence interval,
1.09 to 1.82).
|
The rate of death six months after cardiac arrest was 14 percentage points
lower in the hypothermia group than in the normothermia group (risk
ratio for the hypothermia group, 0.74 [95 percent confidence
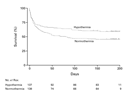
interval, 0.58 to 0.95]) (Table 2 and Figure 2). On
the basis of the difference in the risk of death between the two
groups, 7 patients would need to be treated with hypothermia (95
percent confidence interval, 4 to 33 patients) to prevent 1 death.
After adjustment for base-line differences in the proportions of
patients with a history of diabetes mellitus, a history of coronary
heart disease, and receipt of basic life support from a bystander,
the risk ratio changed only minimally (data not shown). After
adjustment for all the base-line variables shown in Table 1, the
effect of hypothermia on mortality was slightly stronger (risk
ratio, 0.62; 95 percent confidence interval, 0.36 to 0.95).
|
Most of the patients with unfavorable neurologic outcomes died within
six months after discharge from the hospital. In this subgroup of patients,
mortality after discharge did not differ significantly according to
the assigned treatment (Table 3).
|
Complications
The proportion of patients with any complication did not differ
significantly between the two groups (93 of 132 patients in the
normothermia group [70 percent] and 98 of 135 in the hypothermia group
[73 percent], P=0.70). Sepsis was more likely to develop in the
patients with hypothermia than in those with normothermia, although
this difference was not statistically significant (Table 4). The
total number of complications was not significantly higher in the
hypothermia group than in the normothermia group (P=0.09).
|
Discussion
Our results show that among patients in whom spontaneous
circulation had been restored after cardiac arrest due to
ventricular fibrillation, systemic cooling to a bladder temperature
between 32°C and 34°C for 24 hours increased the chance of survival
and of a favorable neurologic outcome (a cerebral-performance
category of 1 or 2), as compared with standard normothermic life
support.
The use of moderate hypothermia after cardiac arrest was initially
reported in the late 1950s and early 1960s.28,29,30
Although the target temperature was lower in these studies than in
ours and the method and duration of cooling also differed from those
in our study, the results were similar. However, the findings were
inconclusive, and the rate of complications was higher than that
observed with the mild hypothermia used in our study. There were no
further investigations of hypothermia as a resuscitative measure
until the 1990s, when laboratory studies demonstrated the benefit of
mild hypothermia.4,5,6,7,8,16
These studies led to preliminary clinical studies of mild
hypothermia.
In the study by Bernard et al.,17
cooling was induced more rapidly (with ice packs) and for a shorter
period than in our study. Nevertheless, the results were similar to
ours. The neurologic outcome has also been consistently favorable in
studies of mild hypothermia in animals.31,32,33,34 In
the pilot studies by Yanagawa et al.18
and Nagao et al.,19
the frequency of a favorable neurologic outcome was similar to that
in our study, although the method and duration of cooling in these
studies differed from those in our study. In contrast to these
encouraging findings, a study of hypothermia in patients with
traumatic brain injury35
showed no improvement in the neurologic outcome. The reasons for this
discrepancy are thought to include the different pathogenesis of
direct central nervous system injury, as well as the late initiation
of cooling in some of the patients and variations in intensive care
and life support among participating hospitals.35,36
Although the proportions of patients with any complication did
not differ significantly between the two treatment groups in our
study, a detailed analysis of the complications and an analysis of
the total number of complications revealed a trend toward a higher
rate of infectious problems in the hypothermia group. Nevertheless,
the benefit of hypothermia exceeded its possible adverse effects.
One limitation of our study was the fact that the attending physicians
could not be blinded to the treatment assignments. The relative risk
may be slightly exaggerated in studies that are not double blind.37
Although the outcome was assessed without knowledge of the treatment
assignments, we did not verify that the blinding was successful.
Even if it was not successful in a few cases, we do not believe that
any bias that might have been introduced would have been strong
enough to invalidate our findings.
The study population was restricted to a group of patients with
a high risk of brain damage because of the specified interval between
the patient's collapse and the first attempt at resuscitation by
emergency medical personnel, as well as other factors, so only 8
percent of the patients assessed for eligibility were included in
the trial. Further studies are warranted to determine whether our
findings apply to patients at lower risk for brain damage and to
those with cardiac arrest due to causes other than ventricular
fibrillation.
Treatment with hypothermia may be of value in terms of public
health. Each year, cardiac arrest occurs in approximately 375,000 people
in Europe,1
about 30,000 of whom would meet our inclusion criteria. We can be 95
percent confident that treatment with hypothermia would prevent an
unfavorable neurologic outcome in 1200 to 7500 of these patients.
Supported by grants from the Biomedicine and Health Programme
(BIOMED 2) implemented under the Fourth RTD Framework Programme 1994–1998
of the European Union (BMH4-CD-96-0667), the Austrian Ministry of
Science and Transport (GZ 5.550/12-Pr/4/95 and GZ
650.0251/2-IV/6/96), and the Austrian Science Foundation (P11405-MED).
K. Heaton and R. Meier (Kinetic Concepts, Wareham, United Kingdom)
provided technical support and the TheraKool cooling device.
We are indebted to the nurses and staff of the participating centers
for their enthusiastic cooperation, to Elaine Ward for editorial
assistance, and to the patients in the study for their trust and
support.
* The investigators who participated in the
Hypothermia after Cardiac Arrest Study Group are listed in the
Appendix.
Source Information
Michael Holzer, M.D., Universitätsklinik für Notfallmedizin, Vienna,
Austria, assumes overall responsibility for the integrity of the report.
Address reprint requests to Dr. Fritz Sterz, Universitätsklinik
für Notfallmedizin, Allgemeines Krankenhaus der Stadt Wien, Währinger Gürtel
18–20/6D, 1090 Vienna, Austria or at [log in to unmask].
References
- de
Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, et al.
Out-of-hospital cardiac arrest in the 1990's: a population-based study in
the Maastricht area on incidence, characteristics and survival. J Am Coll
Cardiol 1997;30:1500-1505.[Medline]
- Negovsky
VA. Postresuscitation disease. Crit Care Med 1988;16:942-946.[Medline]
- Leonov
Y, Sterz F, Safar P, Radovsky A. Moderate hypothermia after cardiac arrest
of 17 minutes in dogs: effect on cerebral and cardiac outcome. Stroke
1990;21:1600-1606.[Abstract]
- Leonov
Y, Sterz F, Safar P, et al. Mild cerebral hypothermia during and after
cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow
Metab 1990;10:57-70.[Medline]
- Sterz F,
Safar P, Tisherman S, Radovsky A, Kuboyama K, Oku K. Mild hypothermic
cardiopulmonary resuscitation improves outcome after prolonged cardiac
arrest in dogs. Crit Care Med 1991;19:379-389.[Medline]
- Weinrauch
V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild
hypothermia and detrimental effect of deep hypothermia after cardiac
arrest in dogs. Stroke 1992;23:1454-1462.[Abstract]
- Kuboyama
K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in
cooling negates the beneficial effect of mild resuscitative cerebral
hypothermia after cardiac arrest in dogs: a prospective, randomized study.
Crit Care Med 1993;21:1348-1358.[Medline]
- Safar P,
Xiao F, Radovsky A, et al. Improved cerebral resuscitation from cardiac
arrest in dogs with mild hypothermia plus blood flow promotion. Stroke
1996;27:105-113.[Abstract/Full
Text]
- Hegnauer
AH, D'Amato HE. Oxygen consumption and cardiac output in the hypothermic
dog. Am J Physiol 1954;178:138-142.
- Mezrow
CK, Sadeghi AM, Gandsas A, et al. Cerebral blood flow and metabolism in
hypothermic circulatory arrest. Ann Thorac Surg 1992;54:609-615.[Abstract]
- Chopp M,
Knight R, Tidwell CD, Helpern JA, Brown E, Welch KM. The metabolic effects
of mild hypothermia on global cerebral ischemia and recirculation in the
cat: comparison to normothermia and hyperthermia. J Cereb Blood Flow Metab
1989;9:141-148.[Medline]
- Dempsey
RJ, Combs DJ, Maley ME, Cowen DE, Roy MW, Donaldson DL. Moderate
hypothermia reduces postischemic edema development and leukotriene
production. Neurosurgery 1987;21:177-181.[Medline]
- Kramer
RS, Sanders AP, Lesage AM, Woodhall B, Sealy WC. The effect of profound
hypothermia on preservation of cerebral ATP content during circulatory
arrest. J Thorac Cardiovasc Surg 1968;56:699-709.[Medline]
- Natale
JA, D'Alecy LG. Protection from cerebral ischemia by brain cooling without
reduced lactate accumulation in dogs. Stroke 1989;20:770-777.[Abstract]
- Sterz F,
Leonov Y, Safar P, et al. Multifocal cerebral blood flow by Xe-CT and
global cerebral metabolism after prolonged cardiac arrest in dogs:
reperfusion with open-chest CPR or cardiopulmonary bypass. Resuscitation
1992;24:27-47.[Medline]
- Busto R,
Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild
hypothermia on ischemia-induced release of neurotransmitters and free
fatty acids in rat brain. Stroke 1989;20:904-910.[Abstract]
- Bernard
SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose
survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997;30:146-153.[Medline]
- Yanagawa
Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild
resuscitative hypothermia after out-of-hospital cardiopulmonary arrest.
Resuscitation 1998;39:61-66.[Medline]
- Nagao K,
Hayashi N, Kanmatsuse K, et al. Cardiopulmonary cerebral resuscitation
using emergency cardiopulmonary bypass, coronary reperfusion therapy and
mild hypothermia in patients with cardiac arrest outside the hospital. J
Am Coll Cardiol 2000;36:776-783.[Medline]
- Zeiner
A, Holzer M, Sterz F, et al. Mild resuscitative hypothermia to improve
neurological outcome after cardiac arrest: a clinical feasibility trial.
Stroke 2000;31:86-94.[Abstract/Full
Text]
- Jennett
B, Bond M. Assessment of outcome after severe brain damage. Lancet
1975;1:480-484.[Medline]
- Brain
Resuscitation Clinical Trial II Study Group. A randomized clinical study
of a calcium-entry blocker (lidoflazine) in the treatment of comatose
survivors of cardiac arrest. N Engl J Med 1991;324:1225-1231.[Abstract]
- Safar P,
Bircher NG. Cardiopulmonary cerebral resuscitation: basic and advanced
cardiac and trauma life support: an introduction to resuscitation
medicine. 3rd ed. London: W.B. Saunders, 1988:267.
- Human
Medicines Evaluation Unit. Note for guidance on good clinical practice
(clinical practice medicinal products [CPMP]/International Conference of
Harmonization [ICH]/135/95). Guidelines for good clinical practice. ICH
topic E6. London: European Agency for the Evaluation of Medicinal
Products, 1995:1-58. (Also available at http://www.eudra.org/emea.html.)
- Cummins
RO, Chamberlain DA, Abramson NS, et al. Recommended guidelines for uniform
reporting of data from out-of-hospital cardiac arrest: the Utstein Style:
a statement for health professionals from a task force of the American
Heart Association, the European Resuscitation Council, the Heart and
Stroke Foundation of Canada, and the Australian Resuscitation Council.
Circulation 1991;84:960-975.[Medline]
- Cuzick
J. A Wilcoxon-type test for trend. Stat Med 1985;4:87-90.[Medline]
- Zhang J,
Yu KF. What's the relative risk? A method of correcting the odds ratio in
cohort studies of common outcomes. JAMA 1998;280:1690-1691.[Medline]
- Benson
DW, Williams GR Jr, Spencer FC, Yates AJ. The use of hypothermia after
cardiac arrest. Anesth Analg 1958;38:423-428.
- Williams
GR Jr, Spencer FC. The clinical use of hypothermia following cardiac
arrest. Ann Surg 1959;148:462-468.
- Ravitch
MM, Lane R, Safar P, Steichen FM, Knowles P. Lightning stroke: report of a
case with recovery after cardiac massage and prolonged artificial
respiration. N Engl J Med 1961;264:36-38.
- Markarian
GZ, Lee JH, Stein DJ, Hong SC. Mild hypothermia: therapeutic window after
experimental cerebral ischemia. Neurosurgery 1996;38:542-550.[Medline]
- Coimbra
C, Wieloch T. Hypothermia ameliorates neuronal survival when induced 2
hours after ischaemia in the rat. Acta Physiol Scand 1992;146:543-544.[Medline]
- Colbourne
F, Li H, Buchan AM. Indefatigable CA1 sector neuroprotection with mild
hypothermia induced 6 hours after severe forebrain ischemia in rats. J
Cereb Blood Flow Metab 1999;19:742-749.[Medline]
- Hickey
RW, Ferimer H, Alexander HL, et al. Delayed, spontaneous hypothermia
reduces neuronal damage after asphyxial cardiac arrest in rats. Crit Care
Med 2000;28:3511-3516.[Medline]
- Clifton
GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia
after acute brain injury. N Engl J Med 2001;344:556-563.[Abstract/Full
Text]
- Safar P,
Kochanek PM. Lack of effect of induction of hypothermia after acute brain
injury. N Engl J Med 2001;345:66-66.[Full
Text]
- Schulz
KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias:
dimensions of methodological quality associated with estimates of
treatment effects in controlled trials. JAMA 1995;273:408-412.[Medline]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.