|
|
Knocking Out the DREAM to Study Pain
|
|
Although some mechanisms by which acute pain evolves into a chronic
syndrome are known, many others, including changes in the brain
stem, thalamus, and cerebral cortex, are not understood. Moreover,
even though chronic neuropathic and inflammatory pain can be
relieved to some extent with opiate compounds, these syndromes are
still poorly understood. The treatment options for pain with a
central cause are even more bleak. Now, however, the revolution in
genomic engineering has opened up new possibilities for studying the
mechanisms of acute and chronic pain and designing novel and more
selective drugs.
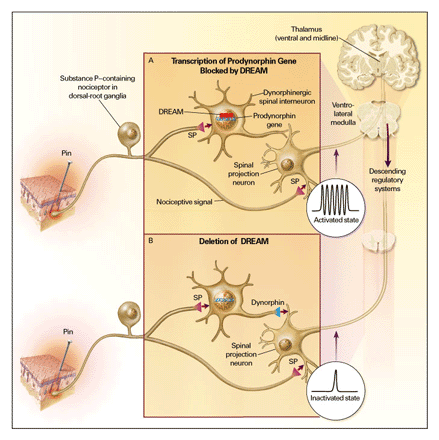
Prodynorphin is the precursor of dynorphin, an opioid
neuropeptide. Expression of the prodynorphin gene is controlled by a
calcium-regulated transcription factor that binds to a downstream
regulatory element (DRE) of the gene and is termed DRE antagonistic
modulator (DREAM). When DREAM binds to DRE, it inhibits
transcription of the prodynorphin gene (Figure 1A).1 In
view of the role of dynorphin in spinal and supraspinal modulation
of afferent nociceptive signals and its likely involvement in
chronic-pain syndromes, the DREAM transcriptional regulator should
be an important target for assessing nociceptive transmission.
|
Cheng et al.2
deleted DREAM in mice by targeted disruption of the gene and
performed a number of groundbreaking studies of the mechanisms by
which DREAM regulates nociception. The DREAM-knockout animals had
essentially normal motor control, spatial learning, and anxiety in
open field tests but significantly reduced responses to acute
thermal and mechanical stimulation of the skin and viscera and
decreased responses in neuropathic and inflammatory models of
chronic pain (Figure
1B). These features make DREAM-knockout mice ideal for assessing
transmitter systems that regulate nociception and reduce pain
behavior mediated by kappa-opioid receptors.
Opioids interact with receptors at all levels of nociceptive and
pain processing. The principal opioid receptors are the mu, kappa,
and delta subtypes. The effectiveness of compounds such as morphine,
which are selective for mu-opioid receptors, reflects the wide
distribution of mu-opioid receptors and the ability of such
compounds to block the transmission of the nociceptor to the spinal
cord and to control the perception of pain in the cerebral cortex.
Dynorphin is released by interneurons in the spinal cord, binds to
kappa-opioid receptors on spinal-projection neurons, and has
analgesic effects. It appears, however, that dynorphin is not
expressed by nociceptors, is elevated in chronic-pain syndromes, and
through its actions at N-methyl-D-aspartate
(NMDA) receptors,3 may
actually enhance nociceptive transmission, under some circumstances,
rather than reduce it. For these reasons, the expression of dynorphins
must be under the tight control of DREAM.
Critical evidence of a specific role of DREAM in pain processing2
was provided by gel-shift assays showing calcium- and dose-dependent
binding of prodynorphin DRE to DREAM and a lack of DREAM in the
DREAM-knockout mice. The DREAM-knockout mice had elevated levels of
messenger RNA (mRNA) for prodynorphin in the spinal cord, normal
levels of mRNA for the opioids pro-opiomelanocortin and proenkephalin
and the DRE-containing c-fos, and no changes in the levels of
kappa-opioid or NMDA receptors in the spinal cord. The decreased
responses to pain in DREAM-knockout mice were mediated by
kappa-opioid receptors, as was shown with the kappa-selective
antagonist norbinaltorphimine dihydrochloride.
These studies also assessed the NMDA receptors in DREAM-knockout
mice. The NMDA receptor mediates the entry of calcium into spinal neurons
through the activation of glutamatergic nociceptors. In the
DREAM-knockout mice, however, a selective NMDA antagonist (MK-801)
had no effect on the tail-flick test, which measures the interval
between noxious heating of the tail and withdrawal of the tail from
the stimulus. Although MK-801 increases the threshold for tail
withdrawal in normal animals, the drug did not change the response
in knockout mice, which suggests that the actions of dynorphin in
the knockout mice are not mediated by an NMDA mechanism, as they are
in normal animals. Since DREAM may also be regulated independently
of nuclear calcium levels,4
the actions of dynorphin in this model may not be directly linked to
central sensitization.
Many new questions arise from these pivotal findings. First, what
is the specificity of DREAM in nociceptive and pain processing? All
pain-induced behavior appears to be interrupted at a nodal point in
the spinal cord of these knockout animals. Although this could prove
to be an important feature of DREAM, it means that descending
inhibitory functions in the brain stem and other functions of
central-pain processing will not be available for study because they
have been permanently blocked. Regulatory specificity is being
uncovered in animals with other knockout targets. For example,
deletion of the dopamine-![]() hydroxylase gene
widely blocks expression of noradrenaline, yet it inhibits hyperalgesia
for thermal but not mechanical stimulation.5
Second, what is the involvement of the supraspinal midbrain and
medial thalamus in altered pain processing in the DREAM-deficient
animals? Although the hippocampus was analyzed for prodynorphin
levels, there was no assessment of pain mechanisms in the forebrain
in regions established as nociceptive. Third, what agents, processes,
and genes regulate the expression of DREAM, and how is it regulated during
acute pain in normal animals? Fourth, how can this gene serve as a
target for therapy? Compounds that regulate the expression of
neuronal genes are not yet available. Indeed, we must evaluate other
systems involved in establishing chronic pain using microarray analysis
in DREAM-knockout animals during acute and chronic pain.
hydroxylase gene
widely blocks expression of noradrenaline, yet it inhibits hyperalgesia
for thermal but not mechanical stimulation.5
Second, what is the involvement of the supraspinal midbrain and
medial thalamus in altered pain processing in the DREAM-deficient
animals? Although the hippocampus was analyzed for prodynorphin
levels, there was no assessment of pain mechanisms in the forebrain
in regions established as nociceptive. Third, what agents, processes,
and genes regulate the expression of DREAM, and how is it regulated during
acute pain in normal animals? Fourth, how can this gene serve as a
target for therapy? Compounds that regulate the expression of
neuronal genes are not yet available. Indeed, we must evaluate other
systems involved in establishing chronic pain using microarray analysis
in DREAM-knockout animals during acute and chronic pain.
There are some difficulties inherent in the use of the
DREAM-knockout model to study pain processing. Since all nociceptive
processing is blocked at a nodal point in the spinal cord, this
model may not tell us much about the unique causes of neuropathic
and inflammatory pain. DREAM-knockout mice express prodynorphin
in the ventral horns, but this has not been observed in normal adult
mice. Moreover, chronic pain usually begins in adulthood, yet the
effect in knockout mice begins very early in life. Further investigation
of these neurons could provide insight into dormant systems that may
be useful for targeting drugs. It should also be noted that chronic
pain reduces nociceptive responses of the anterior insular and
cingulate cortices. Such cortical alterations must be identified in
animal models because these are the sites of pain-induced stress and
anxiety. Although the direct therapeutic value of the knockout model
is limited, targeted delivery of DNA complexes to the central
nervous system is possible.6
The DREAM-knockout model may be useful for designing drugs that
can directly regulate genes, and the side effects of such an approach
may be reduced by the use of site-specific expression vectors. There
is no doubt that merging drug design with genetic engineering has
great potential. The dream is that these engineering techniques will
elucidate the mechanisms of the least understood pain syndromes and
provide selective targets for effective relief of pain, even for the
most intransigent pain syndromes, such as central pain.
Brent A. Vogt, Ph.D.
State University of New York Upstate Medical
University
Syracuse, NY 13210
Supported by a grant (NS38485) from the National Institutes of
Health.
References
- Carrion
AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a CA2+-regulated
transcriptional repressor. Nature 1999;398:80-84.[ISI][Medline]
- Cheng
H-YM, Pitcher GM, Laviolette SR, et al. DREAM is a critical transcriptional
repressor for pain modulation. Cell 2002;108:31-43.[ISI][Medline]
- Laughlin
TM, Larson AA, Wilcox GL. Mechanisms of induction of persistent
nociception by dynorphin. J Pharmacol Exp Ther 2001;299:6-11.[Abstract/Full
Text]
- Ledo F,
Carrion AM, Link WA, Mellstrom B, Naranjo JR. DREAM-
 CREM interaction via leucine-charged domains depresses
downstream regulatory element-dependent transcription. Mol Cell Biol
2000;20:9120-9126.[Abstract/Full
Text]
CREM interaction via leucine-charged domains depresses
downstream regulatory element-dependent transcription. Mol Cell Biol
2000;20:9120-9126.[Abstract/Full
Text]
- Jasmin
L, Tien D, Weinshenker D. et al. The NK1 receptor mediates both the
hyperalgesia and the resistance to morphine in mice lacking noradrenaline.
Proc Natl Acad Sci U S A 2002;99:1029-1034.[Abstract/Full
Text]
- Meuli-Simmem
C, Liu Y, Yeo TT, et al. Gene expression along the cerebral-spinal axis
after regional gene delivery. Hum Gene Ther 1999;10:2689-2700.[ISI][Medline]
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.