|
|
||||||||
|
|
Inhaled Iloprost for Severe Pulmonary
Hypertension
Horst
Olschewski, M.D., Gerald Simonneau, M.D., Nazzareno Galič, M.D., Timothy
Higenbottam, M.D., Robert Naeije, M.D., Lewis J. Rubin, M.D., Sylvia Nikkho,
M.D., Rudolf Speich, M.D., Marius M. Hoeper, M.D., Jürgen Behr, M.D., Jörg
Winkler, M.D., Olivier Sitbon, M.D., Wladimir Popov, M.D., H. Ardeschir
Ghofrani, M.D., Alessandra Manes, M.D., David G. Kiely, M.D., Ralph Ewert, M.D.,
Andreas Meyer, M.D., Paul A. Corris, F.R.C.P., Marion Delcroix, M.D., Miguel
Gomez-Sanchez, M.D., Harald Siedentop, Dipl.Stat., Werner Seeger, M.D., for the
Aerosolized Iloprost Randomized Study Group
ABSTRACT
Background
Uncontrolled studies suggested that aerosolized iloprost, a stable
analogue of prostacyclin, causes selective pulmonary vasodilatation
and improves hemodynamics and exercise capacity in patients with
pulmonary hypertension.
Methods We compared
repeated daily inhalations of 2.5 or 5.0 µg of iloprost (six or nine
times per day; median inhaled dose, 30 µg per day) with inhalation
of placebo. A total of 203 patients with selected forms of severe
pulmonary arterial hypertension and chronic thromboembolic pulmonary
hypertension (New York Heart Association [NYHA] functional class III
or IV) were included. The primary end point was met if, after week
12, the NYHA class and distance walked in six minutes were improved by
at least one class and at least 10 percent, respectively, in the
absence of clinical deterioration according to predefined criteria
and death.
Results The
combined clinical end point was met by 16.8 percent of the patients
receiving iloprost, as compared with 4.9 percent of the patients
receiving placebo (P=0.007). There were increases in the distance
walked in six minutes of 36.4 m in the iloprost group as a whole
(P=0.004) and of 58.8 m in the subgroup of patients with primary
pulmonary hypertension. Overall, 4.0 percent of patients in the
iloprost group (including one who died) and 13.7 percent of those in
the placebo group (including four who died) did not complete the
study (P=0.024); the most common reason for withdrawal was clinical
deterioration. As compared with base-line values, hemodynamic values
were significantly improved at 12 weeks when measured after iloprost
inhalation (P<0.001), were largely unchanged when measured before
iloprost inhalation, and were significantly worse in the placebo
group. Further significant beneficial effects of iloprost treatment
included an improvement in the NYHA class (P=0.03), dyspnea (P=0.015),
and quality of life (P=0.026). Syncope occurred with similar
frequency in the two groups but was more frequently rated as serious
in the iloprost group, although this adverse effect was not
associated with clinical deterioration.
Conclusions Inhaled
iloprost is an effective therapy for patients with severe pulmonary
hypertension.
A continuous infusion of prostacyclin was
the first therapy shown to reduce mortality in a controlled study of
patients with severe pulmonary hypertension.1
However, its use is associated with a number of serious drawbacks.
The lack of pulmonary selectivity results in systemic side effects,
tolerance leads to progressive increases in the dose, and there may
be recurrent infections of the intravenous catheter.2 As
an alternative, inhaled nitric oxide possesses pulmonary
selectivity, but it is less potent than prostacyclin in the pulmonary
vasculature.3,4
Moreover, an interruption in the inhalation of continuous nitric
oxide may cause rebound pulmonary hypertension.5,6
Designed to combine the beneficial effects of prostacyclin with those
of an inhalational application, aerosolized prostacyclin was found
to be a potent pulmonary vasodilator in patients with acute
respiratory failure, exerting preferential vasodilatation in
well-ventilated lung regions.7,8,9,10
Similar results were obtained in spontaneously breathing patients
who had lung fibrosis and severe pulmonary hypertension.11
Iloprost is a stable analogue of prostacyclin that is associated
with a longer duration of vasodilatation.12
When administered during a short aerosolization maneuver to patients
with pulmonary hypertension, its pulmonary vasodilative potency was
similar to that of prostacyclin, but its effects lasted for 30 to 90
minutes, as compared with 15 minutes.4,11,13,14,15 Several
open-label, uncontrolled studies of patients with severe pulmonary
hypertension suggested that long-term use of aerosolized iloprost
results in substantial clinical improvement.11,13,16,17,18,19,20
Our objective in this trial was to evaluate the effects of inhaled
iloprost using a rigorous end point of clinical efficacy.
Methods
Selection of Patients
Patients with primary pulmonary hypertension and selected forms
of nonprimary pulmonary hypertension were candidates for the study.
The forms of nonprimary pulmonary hypertension included appetite-suppressant–associated
and scleroderma-associated pulmonary hypertension as well as
inoperable chronic thromboembolic pulmonary hypertension. The
inclusion criteria were a mean pulmonary-artery pressure greater
than 30 mm Hg, the ability to cover between 50 and 500 m without
encouragement on a six-minute walk test,21
and a New York Heart Association (NYHA) functional class of III
or IV22
despite the use of standard conventional therapy (anticoagulants,
diuretics, digitalis, calcium-channel blockers, and supplemental
oxygen). Patients who were taking investigational drugs,
prostanoids, or beta-blockers were excluded. The doses of
calcium-channel blockers had to be constant for more than six weeks
before study entry. Exclusion criteria were a pulmonary-artery wedge
pressure at rest of more than 15 mm Hg, a cardiac index at rest of
less than 1.5 or more than 4 liters per minute per square meter of
body-surface area, bleeding disorders, a bilirubin level of more
than 3 mg per deciliter (51 µmol per liter), creatinine clearance
below 30 ml per minute, a forced vital capacity below 50 percent, a
forced expiratory volume in one second that was less than the mean
normal value minus twice the standard deviation, and clinical
instability.
Study Design
A total of 203 patients participated after giving written informed
consent and after the study had been approved by the local ethics committees
at 37 European specialist centers. Patients were randomly assigned
to receive iloprost (Ilomedin, Schering) or placebo after
stratification according to NYHA functional class (III or IV) and
type of pulmonary hypertension (primary or nonprimary) by an
independent committee whose members were unaware of patients' identities.
A total of 101 patients were randomly assigned to the iloprost
group, and 102 were assigned to the placebo group.
For inhalation, iloprost or placebo was diluted with saline to
a concentration of 10 µg per milliliter, and 2 ml was added to a
nebulizer (HaloLite, MedicAid). This device delivered short pulses
of aerosolized particles (geometric median [±SD] aerodynamic diameter
of particles, 4.3±0.05 µm)23
during the first part of each inspiration until a predefined total
inhaled dose of 2.5 µg had been dispensed. The inhalation was then
stopped or repeated once, to achieve a total dose of 5.0 µg,
depending on how well the patient tolerated the treatment. After
each inhalation, the residual volume in the nebulizer was discarded.
This maneuver was repeated six or nine times daily, with an
overnight break. The frequency of inhalation and the dose were
individually determined within the first eight days of therapy
according to a predefined dosing algorithm.
Right-heart catheterization was performed in all patients at base
line and after 12 weeks. The acute effects of inhaled iloprost were
evaluated after 12 weeks in all patients, but not at base line, to
avert unblinding. At base line and after 4, 8, and 12 weeks,
patients completed a six-minute walk test, the Mahler Dyspnea Index
questionnaire,24
the EuroQol questionnaire,25
and the 12-item Medical Outcomes Study Short-Form General Health Survey.26
Patients were removed from the study if they met two or more of
the following predefined criteria for a deterioration in their
condition: refractory systolic arterial hypotension (blood pressure,
less than 85 mm Hg); worsening right ventricular failure (e.g., as
indicated by the development of refractory edema or ascites);
rapidly progressing cardiogenic, hepatic, or renal failure; a
decrease of at least 30 percent in the distance walked in six
minutes; and a decline in measures of hemodynamic function, such as
central venous pressure and mixed venous oxygen saturation.
Outcome Measures
The primary end point of the study consisted of an increase of
at least 10 percent in the distance walked in six minutes and an
improvement in the NYHA functional class in the absence of a
deterioration in the clinical condition or death during the 12 weeks
of the study. Secondary efficacy variables were changes in the
values for the six-minute walk test, the NYHA class, Mahler Dyspnea
Index scores, hemodynamic variables, and the quality of life;
clinical deterioration; death; and the need for transplantation.
Statistical Analysis
The primary evaluation of efficacy included all randomized
patients. Data are presented as means ±SD, unless otherwise stated.
We included data on patients who prematurely discontinued the study
using a last-observation-carried-forward analysis for the six-minute
walk test. Patients who died were assigned a value of 0 m. All
statistical tests for efficacy variables were two-tailed, with an
alpha level of 0.05.
To analyze the primary efficacy end point and the improvement
criteria, we used the Mantel–Haenszel test,27
stratified according to the type of pulmonary hypertension (primary
or nonprimary) and NYHA class (III or IV). Patients with missing
data on the primary end point at week 12 were considered not to
have had a response.
Changes in the results of the six-minute walk were evaluated with
use of nonparametric analysis of covariance stratified according to
the type of pulmonary hypertension (primary or nonprimary) and the
NYHA class (III or IV), with use of the base-line value as the
covariate (analysis of covariance), and the Wilcoxon signed-rank
test.
Changes from base line in hemodynamic values were analyzed with
t-statistics. The investigators had full access to the data and
performed the analyses independently of the sponsor.
Results
Base-line demographic and hemodynamic data are given in Table 1. The
mean frequency of inhalation was 7.5 times per day. Ninety-one percent
of patients received 5.0 µg per inhalation, and 9 percent received
2.5 µg, corresponding to a median inhaled dose of 30 µg per day.
|
Primary Efficacy End Point
For the primary end point, we found a significant effect of treatment
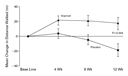
in favor of iloprost (P=0.007) (Figure 1). The
estimated odds of an effect in the iloprost group, as compared with
the placebo group, were 3.97 (95 percent confidence interval, 1.47
to 10.75, by the Mantel–Haenszel test), with no significant heterogeneity
among the four subgroups categorized according to type of pulmonary
hypertension and NYHA class at base line (P=0.79 by the Breslow–Day
test). The secondary analysis of the primary end point was a
logistic-regression model that included treatment assignment,
demographic data, and base-line characteristics. Only treatment
assignment (P=0.01) contributed significantly to the probability of
a response.
|
Secondary End Points
Six-Minute
Walk Test
The percentage of patients who had an increase of at least 10
percent in the distance walked in six minutes at week 12 was slightly,
but not significantly, higher in the iloprost group than in the
placebo group (P=0.06) (Table 2). The
type of pulmonary hypertension had no significant effect on the
outcome in either group (P=0.90). A higher percentage of patients in
the placebo group than in the iloprost group had a decrease in the
distance walked of at least 10 percent or did not complete the study
(Table 2).
|
The absolute change in the distance walked in six minutes was significantly
larger (by 36.4 m) in the iloprost group than in the placebo group
(P=0.004) (Figure
1): 58.8 m among those with primary pulmonary hypertension and
12 m among those with nonprimary pulmonary hypertension. A
parametric analysis of covariance, which included the absolute value
on the six-minute walk test at week 12 as a dependent variable and
the treatment assignment (P=0.02), type of pulmonary hypertension
(P=0.06), and distance walked at base line (P<0.001) did not show
a statistically significant interaction between treatment and type
of pulmonary hypertension (P=0.09).
NYHA Class
More patients in the iloprost group than in the placebo group
had an improvement in the severity of heart failure, as assessed by
the NYHA class (P=0.03) (Table 2). The
type of pulmonary hypertension had no effect on the outcome in
either group (P=0.39). The percentage of patients with a
deterioration in NYHA class did not differ significantly between the
groups, but the analysis did not include patients who left the study
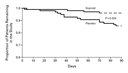
early owing to death or other reasons. A larger proportion of
patients in the placebo group than in the iloprost group did not
complete the study (Table 2 and Figure 2).
Reasons included death, discontinuation of study medication, and
withdrawal of consent, mostly owing to clinical deterioration,
insufficient clinical benefit, or both.
|
Hemodynamics and Gas Exchange
In the placebo group, cardiac output, systemic arterial oxygen
saturation, and mixed venous oxygen saturation decreased significantly
after 12 weeks and pulmonary vascular resistance and right atrial pressure
increased significantly (Table 3). In the
iloprost group, values assessed at 12 weeks, before the first
morning dose of inhaled iloprost, were largely unchanged from base
line, whereas values assessed after inhalation were significantly
decreased (in the case of pulmonary-artery pressure, pulmonary vascular
resistance, systemic arterial pressure, and systemic arterial oxygen
saturation) or increased (in the case of carbon monoxide and
pulmonary-artery wedge pressure). At the completion of the 12-week
study, acute hemodynamic responsiveness to inhaled iloprost was
equivalent in the placebo group and the iloprost group, though the
latter group had been exposed to daily iloprost inhalation for three
months (data not shown).
|
Mahler Dyspnea Index
The mean Mahler Dyspnea Index transition score was significantly
better at week 12 in the iloprost group than in the placebo group
(change, +1.42±2.59 vs. +0.30±2.45; P=0.015). The type of pulmonary
hypertension had no effect on this outcome.
Quality of Life
Mean scores on the EuroQol visual-analogue scale improved
significantly (from 46.9±15.9 to 52.8±19.1) in the iloprost group
but were virtually unchanged in the placebo group (dropping from
48.6±16.9 to 47.4±21.1, P=0.026 by analysis of covariance). The
EuroQol health-state score improved from 0.49±0.28 to 0.58±0.27 in
the iloprost group and was unchanged in the placebo group (0.56±0.29
to 0.56±0.31, P=0.11 by analysis of covariance). None of the other
measures of the quality of life were significantly different between
the groups.
Clinical Deterioration and Death
One patient died in the iloprost group during the 12-week study,
as compared with four patients in the placebo group (P=0.37) (Table 2).
Criteria for clinical deterioration were met in 4.9 percent of
patients in the iloprost group and 8.8 percent of those in the
placebo group (P=0.41). This indicated that fewer patients either
died or deteriorated in the iloprost group than in the placebo group
(4.9 percent vs. 11.8 percent, P=0.09). The type of pulmonary
hypertension had no effect on the outcome. During the study period,
none of the patients received a lung transplant.
Safety
The total number of patients who had serious adverse events did
not differ significantly between the groups (Table 4). Right
ventricular failure and edema were more than twice as frequent in
the placebo group as in the iloprost group. The total number of
syncopal events in each of the two groups was similar (eight in the
iloprost group and five in the placebo group), but these events were
more often considered serious in the iloprost group. Syncope was not
associated with clinical deterioration or premature withdrawal from
the study. Syncopal events occurred more than two hours after the
last inhalation (often after an overnight break), were
exercise-induced in two patients, were induced by bradycardia in two
patients (associated with gastroenteritis in one patient and with
verapamil therapy in the other), and resulted in head trauma in one
patient. Flushing and jaw pain were more common in the iloprost
group, but these adverse effects were mostly transient and mild and
were not considered to be serious in any patient.
|
Discussion
The results of this clinical trial demonstrate that long-term
inhaled administration of aerosolized iloprost, a stable analogue of
prostacyclin, improves a clinically important combined end point
consisting of exercise capacity, NYHA class, and clinical deterioration
in patients with selected forms of pulmonary arterial hypertension
and chronic thromboembolic pulmonary hypertension. Moreover,
iloprost improved several secondary end points.
Since intravenous epoprostenol was shown to improve survival among
the most severely ill patients with primary pulmonary hypertension,
it has been unethical to perform randomized clinical trials among
patients with pulmonary hypertension in which survival is used as an
end point. We chose a combined rather than a single end point (e.g.,
the distance walked in six minutes) in order to make a more rigorous
determination of whether inhaled iloprost was efficacious. Nearly 40
percent of all patients who were treated with iloprost increased
their six-minute walking distance by at least 10 percent. However,
only half as many patients also had improvement in the NYHA class;
conversely, not all patients with an improvement in NYHA class had
an increase of at least 10 percent in the distance walked in six
minutes. Thus, although only 17 percent of patients in the iloprost
group reached the combined end point, a substantial number of the
remaining patients met less strict criteria for clinical improvement
that would warrant continued therapy. Furthermore, significantly
fewer patients in the iloprost group than in the placebo group prematurely
discontinued the study as a result of lack of efficacy or other
reasons, suggesting that even when iloprost therapy does not produce
substantial improvement, it may stabilize the clinical condition.
The mean inhaled dose of iloprost corresponded to 0.37 ng per
kilogram of body weight per minute, which is considerably lower than
an effective intravenous or subcutaneous dose.2,28
Thus, targeted delivery of prostanoids to the pulmonary vasculature
by means of inhalation may substantially reduce the drug requirements.
Like other investigators, we found that the benefit was greatest
among patients with primary pulmonary hypertension and was similar to
that of epoprostenol1
and bosentan.29
Although patients with nonprimary pulmonary hypertension had
improvement in the scores for the Mahler Dyspnea Index and
quality-of-life measures that were similar to those achieved in
patients with primary pulmonary hypertension, fewer such patients
reached the combined end point, and they also had a smaller absolute
change in the distance walked in six minutes. Similar results have
been obtained with the use of other drugs for pulmonary
hypertension, including epoprostenol,30
beraprost,31
and treprostinil.28
Hemodynamic assessments of preinhalation values showed that values
stabilized in the iloprost group, whereas they deteriorated in the
placebo group. The degree of deterioration may be underestimated, since
patients who discontinued treatment prematurely did not undergo
follow-up hemodynamic examination. Postinhalation assessments of
hemodynamic variables demonstrated a significant improvement in the
iloprost group, as was anticipated on the basis of previous reports.4,11,13,16
Since the acute hemodynamic response did not differ between the
groups, it appears unlikely that tolerance developed over the
12-week course of iloprost treatment. During long-term treatment,
the patients' hemodynamic status is somewhere between preinhalation
and postinhalation values. In comparison, continuous intravenous
therapy may result in a more sustained hemodynamic improvement32;
however, continuous intravenous therapy also poses considerable
risks, including relapse after the interruption of therapy and
complications, and is difficult to administer.
With respect to adverse events, flushing was more common in the
iloprost group, but the frequency of most of the other inhalation-associated
side effects was similar. There were more syncopal episodes in
the iloprost group than in the placebo group (eight vs. five), and
these episodes were more frequently defined as serious adverse events,
but they were not associated with clinical deterioration. Since
syncope occurred a relatively long time (two to nine hours) after
the last inhalation, the loss of an effect of iloprost may have
caused these events. However, the same side effect was observed with
bosentan therapy, suggesting that these drugs may have a more
pronounced effect on blood pressure during exercise. Alternatively,
patients who had clinical improvement with therapy may have become
more physically active, challenging the limits of their cardiac
reserve. We would advise patients to increase their physical
activity gradually after the initiation of therapy for pulmonary
hypertension.
The inhalation device that we used provided accurate doses of
iloprost. However, it is not battery-driven, and inhalation commonly
required 10 minutes. Different techniques of administering aerosolized
iloprost result in similar acute hemodynamic effects as long as
identical doses are delivered to the respiratory tract in a particle
size suitable for alveolar deposition.14,33
With other techniques, the duration of inhalation may be shortened considerably.14
In conclusion, this large, placebo-controlled trial demonstrates
the efficacy and safety of inhaled iloprost for the treatment of
severe primary pulmonary hypertension and selected forms of
pulmonary arterial and chronic thromboembolic pulmonary hypertension. The
advantages of intermittent inhaled therapy over intravenous therapy,
coupled with the improvement in a number of clinically meaningful
variables, suggest that inhaled iloprost therapy is effective. It
may be a suitable alternative to continuous intravenous
prostacyclin, especially in patients who do not derive a clear
survival benefit with intravenous therapy.
Supported by
Schering, Berlin, Germany. All the authors have financial
relationships with Schering, the sponsor of the study. The
relationships differ among the authors and include employment, consultancy,
membership in the scientific advisory board, and support for work as
investigators.
* The other members of the Aerosolized
Iloprost Randomized (AIR) study group are listed in the Appendix.
Source Information
From the Department of Internal Medicine II, University Clinic,
Giessen, Germany (H.O., H.A.G., W.S.); Service de Pneumologie, Hōpital Antoine
Béclčre, Clamart, France (G.S., O.S.); Istituto di Cardiologia, Universitą di
Bologna, Bologna, Italy (N.G., A.M.); Royal Hallamshire Hospital, Sheffield,
United Kingdom (T.H., D.G.K.); Department of Cardiology, Erasme University
Hospital, Brussels, Belgium (R.N.); University of California at San Diego
Medical Center, La Jolla (L.J.R.); Schering, Berlin, Germany (S.N., H.S.);
Department of Internal Medicine, University Hospital, Zurich, Switzerland
(R.S., W.P.); Department of Pneumology, Hannover Medical School, Hannover,
Germany (M.M.H.); the Division of Pulmonary Diseases, University of
Munich–Großhadern, Munich, Germany (J.B.); Department of Pneumology, University
Clinic, Leipzig, Germany (J.W.); Department of Pneumology and Infectious
Diseases, Ernst Moritz Arndt University, Greisswald, Germany (R.E.); Bereich
Pneumologie, Medizinische Kernklinik und Poliklinik, Universitätsklinikum
Hamburg-Eppendorf, Hamburg, Germany (A.M.); Freeman Hospital, High Heaton,
Newcastle-upon-Tyne, United Kingdom (P.A.C.); Department of Pneumology,
Gasthuisberg University Clinic, Leuven, Belgium (M.D.); and Pulmonary
Hypertension Unit, Hospital 12 de Octubre, Madrid (M.G.-S.).
Address reprint requests to Dr. Seeger at the Department of
Internal Medicine II, University Clinic, Klinikstr. 36, D-35392 Giessen,
Germany, or at [log in to unmask].
References
- Barst
RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous
epoprostenol (prostacyclin) with conventional therapy for primary
pulmonary hypertension. N Engl J Med 1996;334:296-301.[Abstract/Full
Text]
- Barst
RJ, Rubin LJ, McGoon MD, Caldwell EJ, Long WA, Levy PS. Survival in
primary pulmonary hypertension with long-term continuous intravenous prostacyclin.
Ann Intern Med 1994;121:409-415.[ISI][Medline]
- Warren
JB, Higenbottam T. Caution with use of inhaled nitric oxide. Lancet
1996;348:629-630.[ISI][Medline]
- Hoeper
MM, Olschewski H, Ghofrani HA, et al. A comparison of the acute
hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in
primary pulmonary hypertension. J Am Coll Cardiol 2000;35:176-182.[ISI][Medline]
- Miller
OI, Tang SF, Keech A, Celermajer DS. Rebound pulmonary hypertension on
withdrawal from inhaled nitric oxide. Lancet 1995;346:51-52.[ISI]
- Cueto E,
Lopez-Herce J, Sanchez A, Carrillo A. Life-threatening effects on
discontinuing inhaled nitric oxide in children. Acta Paediatr
1997;86:1337-1339.[ISI][Medline]
- Walmrath
D, Schneider T, Pilch J, Grimminger F, Seeger W. Aerosolized prostacyclin
in adult respiratory distress syndrome. Lancet 1993;342:961-962.[ISI][Medline]
- Walmrath
D, Schneider T, Pilch J, Schermuly R, Grimminger F, Seeger W. Effects of
aerosolized prostacyclin in severe pneumonia: impact of fibrosis. Am J
Respir Crit Care Med 1995;151:724-730.[Abstract]
- Walmrath
D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W. Direct
comparison of inhaled nitric oxide and aerosolized prostacyclin in acute
respiratory distress syndrome. Am J Respir Crit Care Med 1996;153:991-996.[Abstract]
- Zwissler
B, Kemming G, Habler O, et al. Inhaled prostacyclin (PGI2)
versus inhaled nitric oxide in adult respiratory distress syndrome. Am J
Respir Crit Care Med 1996;154:1671-1677.[Abstract]
- Olschewski
H, Ghofrani HA, Walmrath D, et al. Inhaled prostacyclin and iloprost in
severe pulmonary hypertension secondary to lung fibrosis. Am J Respir Crit
Care Med 1999;160:600-607.[Abstract/Full
Text]
- Fitscha
P, Tiso B, Krais T, Sinzinger H. Effect of iloprost on in vivo and in
vitro platelet function in patients with peripheral vascular disease
(PVD). Adv Prostaglandin Thromboxane Leukot Res 1987;17:450-454.
- Olschewski
H, Walmrath D, Schermuly R, Ghofrani HA, Grimminger F, Seeger W.
Aerosolized prostacyclin and iloprost in severe pulmonary hypertension.
Ann Intern Med 1996;124:820-824.[ISI][Medline]
- Gessler
T, Schmehl T, Hoeper MM, et al. Ultrasonic versus jet nebulization of
iloprost in severe pulmonary hypertension. Eur Respir J 2001;17:14-19.[ISI][Medline]
- Wensel
R, Opitz CF, Ewert R, Bruch L, Kleber FX. Effects of iloprost inhalation
on exercise capacity and ventilatory efficiency in patients with primary
pulmonary hypertension. Circulation 2000;101:2388-2392.[Abstract/Full
Text]
- Hoeper
MM, Schwarze M, Ehlerding S, et al. Long-term treatment of primary
pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue.
N Engl J Med 2000;342:1866-1870.[Abstract/Full
Text]
- Olschewski
H, Ghofrani HA, Walmrath D, Temmesfeld-Wollbrück B, Grimminger F, Seeger
W. Recovery from circulatory shock in severe primary pulmonary
hypertension (PPH) with aerosolization of iloprost. Intensive Care Med
1998;24:631-634.[ISI][Medline]
- Stricker
H, Domenighetti G, Fiori G, Mombelli G. Sustained improvement of
performance and haemodynamics with long-term aerosolised prostacyclin
therapy in severe pulmonary hypertension. Schweiz Med Wochenschr
1999;129:923-927.[ISI][Medline]
- Olschewski
H, Ghofrani HA, Schmehl T, et al. Inhaled iloprost to treat severe
pulmonary hypertension: an uncontrolled trial. Ann Intern Med
2000;132:435-443.[ISI][Medline]
- Beghetti
M, Berner M, Rimensberger PC. Long term inhalation of iloprost in a child
with primary pulmonary hypertension: an alternative to continuous
infusion. Heart 2001;86:E10-E10.[Medline]
- Guyatt
GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of
exercise capacity in patients with chronic heart failure. Can Med Assoc J
1985;132:919-923.[ISI][Medline]
- Rich S,
ed. Primary pulmonary hypertension: executive summary from the World
Symposium — Primary Pulmonary Hypertension 1998. Evian, France: World
Health Organization, 1998. (Accessed July 8, 2002, at http://www.who.int/ncd/cvd/pph.html.)
- Roth C,
Heyder J, Wagner T, Schmehl T, Gessler T. In vitro characterization of
Ilomedin inhalation devices. J Aerosol Med 1999;12:98-98.abstract
- Mahler
DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea:
contents, interobserver agreement, and physiologic correlates of two new
clinical indexes. Chest 1984;85:751-758.[Abstract]
- EuroQol
-- a new facility for the measurement of health-related quality of life.
Health Policy 1990;16:199-208.[ISI][Medline]
- Ware JE,
Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I.
Conceptual framework and item selection. Med Care 1992;30:473-483.[ISI][Medline]
- Mantel
N, Haenszel W. Statistical aspects of the analysis of data from
retrospective studies of disease. J Natl Cancer Inst 1959;22:719-748.[ISI]
- Simonneau
G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of
treprostinil, a prostacyclin analogue, in patients with pulmonary arterial
hypertension: a double-blind, randomized, placebo-controlled trial. Am J
Respir Crit Care Med 2002;165:800-804.[Abstract/Full
Text]
- Rubin
LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial
hypertension. N Engl J Med 2002;346:896-903. [Erratum, N Engl J Med
2002;346:1258.][Abstract/Full
Text]
- Badesch
DB, Tapson VF, McGoon MD, et al. Continuous intravenous epoprostenol for
pulmonary hypertension due to the scleroderma spectrum of disease: a
randomized, controlled trial. Ann Intern Med 2000;132:425-434.[ISI][Medline]
- Galie N,
Humbert M, Vachiéry JL, et al. Effects of beraprost sodium, an oral
prostacyclin analogue, in patients with pulmonary arterial hypertension: a
randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol
2002;39:1496-1502.[ISI][Medline]
- McLaughlin
VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular
resistance with long-term epoprostenol (prostacyclin) therapy in primary
pulmonary hypertension. N Engl J Med 1998;338:273-277.[Abstract/Full
Text]
- Olschewski
H, Rohde B, Behr J, et al. Comparison of inhalation of the prostacyclin
analogue iloprost using different nebulizer systems in patients suffering
from pulmonary hypertension. Chest 2000;118:Suppl:136S-136S.abstract
Edward E.
Rylander, M.D.
Diplomat American
Board of Family Practice.
Diplomat American
Board of Palliative Medicine.